Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2017-10-21 , DOI: 10.1016/j.bmc.2017.10.033 Shaopeng Wei , Li Li , Yaping Shu , Kun Zhao , Zhiqin Ji
|
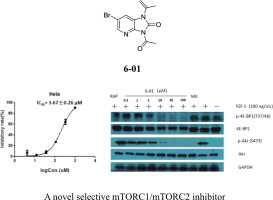
Thirty-six imidazolin-2-ones, including ten pairs of benzimidazolones and sixteen imidazopyridines, were synthesized and subjected for the evaluation of antifungal and antitumor activity. Compounds 4a-01, 6-01, 6-04 and 6-06 could effectively inhibit the spore germination and mycelium growth of Botrytis cinerea. The relationship between structure and antifungal activity revealed that the introducing short-chain aliphatic acyl groups at the moiety of imidazopyridines is favorable for the antifungal activity, whereas aromatic acyl groups are much better than aliphatic acyl groups for the activity of benzimidazolones except for acetyl. Preliminary SRB assay indicated that 6-01 exerted strong antiproliferative effect against Hela and NCM460 cell lines. Further kinases assay revealed that 6-01 could specially inhibit mTOR among 114 human cancer related kinases. Elisa and Western blot analysis testified that 6-01 simultaneously inhibits the phosphorylation of Akt and 4E-BP1, and 6-01 is a novel mTOR inhibitor which targets on both mTORC1 and mTORC2. This investigation provided a valuable chemical structure for the development of antitumor drugs.
中文翻译:

两种新型咪唑啉-2-酮的合成,抗真菌和抗肿瘤活性
合成了三十六个咪唑啉-2-酮,包括十对苯并咪唑酮和十六个咪唑并吡啶,并进行了抗真菌和抗肿瘤活性的评估。化合物4A-01,6-01,6 - 04和6 - 06能有效抑制孢子萌发和菌丝体生长灰霉病。结构与抗真菌活性之间的关系表明,在咪唑并吡啶的部分引入短链脂肪族酰基对于抗真菌活性是有利的,而除了乙酰基以外,芳香族酰基对苯并咪唑啉酮的活性要比脂肪族酰基好得多。初步SRB分析表明,6 - 01施加针对Hela细胞和NCM460细胞系强的抗增殖作用。此外激酶分析的结果,6 - 01所能间114种人类癌症相关激酶专门抑制mTOR。ELISA和Western印迹分析证实,6 - 01同时抑制Akt和4E-BP1,和磷酸化6- 01是一种新的mTOR抑制剂两者的mTORC1和mTORC2哪个目标。这项研究为开发抗肿瘤药物提供了有价值的化学结构。











































 京公网安备 11010802027423号
京公网安备 11010802027423号