Journal of Catalysis ( IF 6.5 ) Pub Date : 2017-10-15 , DOI: 10.1016/j.jcat.2017.09.013 Shusaku Torii , Keiko Jimura , Shigenobu Hayashi , Ryuji Kikuchi , Atsushi Takagaki
|
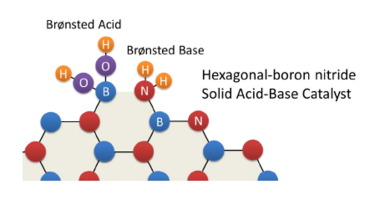
This work explores the use of hexagonal boron nitride (h-BN), a graphite-like compound, as a novel catalyst with base and acid functionalities. For use as a solid catalyst, the layered structure of h-BN was disrupted by ball-milling, exposing boron and nitrogen edge sites as well as increasing the surface area from 3 to ca. 400 m2 g−1. Fourier transform infrared spectroscopy, X-ray photoelectron spectroscopy, and proton magic-angle spinning nuclear magnetic resonance spectroscopy (1H MAS NMR) indicated simultaneous and adjacent formation of amino and hydroxyl groups by milling, which function as Brønsted base and acid sites, respectively. Analysis using color indicator reagents and pyrrole-adsorbed 1H MAS NMR results revealed that the ball-milled h-BN had basic sites of strength +9.3 > H− ≥ +7.2, comparable to those of KY zeolite. Measurements of 31P MAS NMR of adsorbed trimethylphosphine oxide indicated that the ball-milled h-BN had weak acid sites, comparable to those in HY zeolite. Despite its weak basicity, the ball-milled h-BN showed high activity and selectivity toward β-nitroalkenes for the nitroaldol reaction (Henry reaction) and the Knoevenagel condensation, whereas nontreated h-BN did not show activity. The nitroaldol reaction was considered to proceed in two steps: the abstraction of a proton from nitromethane by the amino group and the formation of an imine followed by a nucleophilic attack of the deprotonated nitromethane. Kinetic isotope effect experiments using D-substituted nitromethane revealed that the first step was the rate-determining step. Several nitroaldol reactions using a variety of monosubstituted benzaldehydes indicated that electron-donating groups enhanced the activity, suggesting that the formation of adjacent base and acid sites is responsible for it. This study shows the high catalytic activity of BN, a solid catalyst with moderate basicity and weak acidity.
中文翻译:

六方氮化硼作为固体酸碱双功能催化剂的用途
这项工作探索了使用六方氮化硼(h-BN)(一种类石墨化合物)作为具有碱和酸官能团的新型催化剂的用途。为了用作固体催化剂,h-BN的层状结构通过球磨破坏,暴露出硼和氮的边缘位点,并将表面积从3增加到大约1。400 m 2 g -1。傅里叶变换红外光谱,X射线光电子能谱和质子魔术角旋转核磁共振光谱(1 H MAS NMR)表明,通过研磨同时和相邻形成氨基和羟基,分别用作布朗斯台德碱位和酸性位。使用颜色指示剂和吡咯吸附剂进行分析1H MAS NMR结果表明,球磨h-BN具有+9.3> H - ≥+7.2的碱性位点,与KY沸石相当。吸附的三甲基氧化膦的31 P MAS NMR测定表明,与HY沸石相比,球磨h-BN具有弱酸位。尽管其碱性弱,但球磨的h-BN仍具有较高的活性和对硝基硝基醇反应(亨利反应)和Knoevenagel缩合反应对β-硝基烯烃的选择性,而未处理的h-BN则未显示活性。认为硝基羟醛反应分两个步骤进行:通过氨基从硝基甲烷提取质子,形成亚胺,然后对去质子化的硝基甲烷进行亲核攻击。动力学同位素效应实验D-取代的硝基甲烷表明第一步是速率确定步骤。使用多种单取代的苯甲醛进行的几次硝基羟醛反应表明,供电子基团增强了该活性,表明形成邻近的碱基和酸位是其原因。这项研究表明BN具有高的催化活性,这是一种具有中等碱性和弱酸性的固体催化剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号