Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Activity and safety of AZD3759 in EGFR-mutant non-small-cell lung cancer with CNS metastases (BLOOM): a phase 1, open-label, dose-escalation and dose-expansion study.
The Lancet ( IF 98.4 ) Pub Date : 2017-11-01 , DOI: 10.1016/s2213-2600(17)30378-8 Myung-Ju Ahn , Dong-Wan Kim , Byoung Chul Cho , Sang-We Kim , Jong Seok Lee , Jin-Seok Ahn , Tae Min Kim , Chia-Chi Lin , Hye Ryun Kim , Thomas John , Steven Kao , Jonathan W Goldman , Wu-Chou Su , Ronald Natale , Sarit Rabbie , Bryony Harrop , Philip Overend , Zhenfan Yang , James Chih-Hsin Yang
The Lancet ( IF 98.4 ) Pub Date : 2017-11-01 , DOI: 10.1016/s2213-2600(17)30378-8 Myung-Ju Ahn , Dong-Wan Kim , Byoung Chul Cho , Sang-We Kim , Jong Seok Lee , Jin-Seok Ahn , Tae Min Kim , Chia-Chi Lin , Hye Ryun Kim , Thomas John , Steven Kao , Jonathan W Goldman , Wu-Chou Su , Ronald Natale , Sarit Rabbie , Bryony Harrop , Philip Overend , Zhenfan Yang , James Chih-Hsin Yang
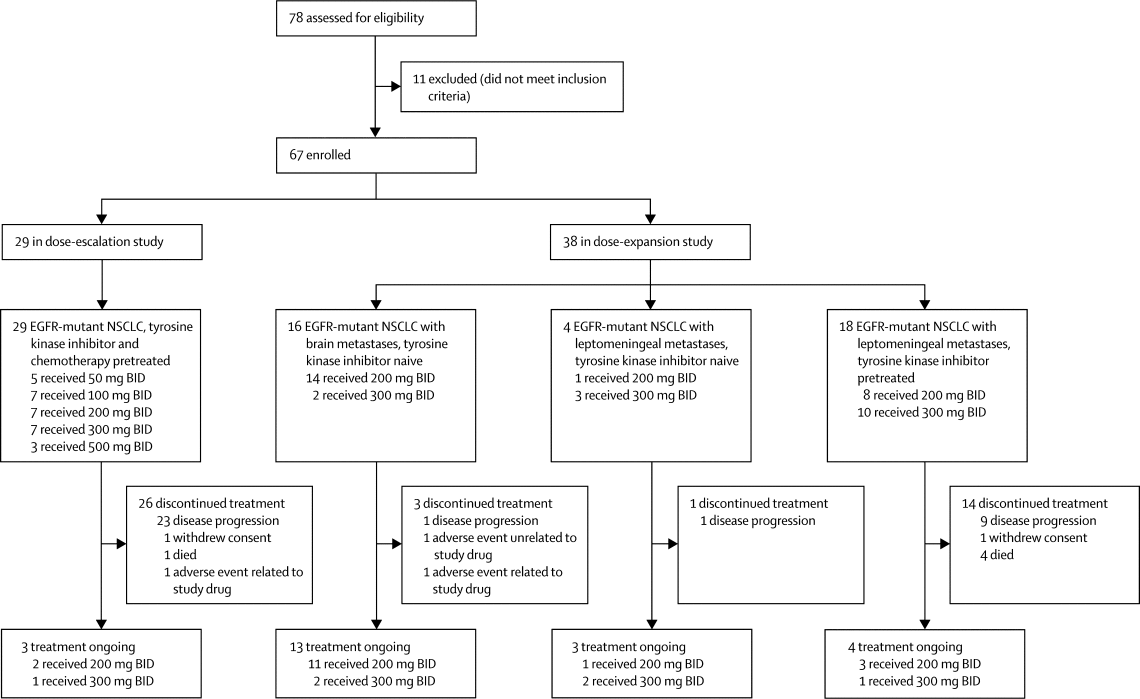
|
CNS metastases-including brain and leptomeningeal metastases-from epidermal growth factor receptor (EGFR)-mutant non-small-cell lung cancer (NSCLC) are associated with poor prognosis. AZD3759 is a novel EGFR tyrosine kinase inhibitor with high capability to penetrate the blood-brain barrier. We aimed to assess the safety, tolerability, pharmacokinetics, and efficacy of AZD3759 in patients with EGFR-mutant NSCLC with brain and leptomeningeal metastases.
中文翻译:

AZD3759在EGFR突变的具有CNS转移的非小细胞肺癌(BLOOM)中的活性和安全性:阶段1,开放标签,剂量递增和剂量扩展研究。
表皮生长因子受体(EGFR)突变的非小细胞肺癌(NSCLC)引起的中枢神经系统转移,包括脑和软脑膜转移,与预后不良相关。AZD3759是一种新型的EGFR酪氨酸激酶抑制剂,具有很高的穿透血脑屏障的能力。我们旨在评估AZD3759在具有脑和软脑膜转移的EGFR突变型NSCLC患者中的安全性,耐受性,药代动力学和疗效。
更新日期:2017-10-20
中文翻译:

AZD3759在EGFR突变的具有CNS转移的非小细胞肺癌(BLOOM)中的活性和安全性:阶段1,开放标签,剂量递增和剂量扩展研究。
表皮生长因子受体(EGFR)突变的非小细胞肺癌(NSCLC)引起的中枢神经系统转移,包括脑和软脑膜转移,与预后不良相关。AZD3759是一种新型的EGFR酪氨酸激酶抑制剂,具有很高的穿透血脑屏障的能力。我们旨在评估AZD3759在具有脑和软脑膜转移的EGFR突变型NSCLC患者中的安全性,耐受性,药代动力学和疗效。











































 京公网安备 11010802027423号
京公网安备 11010802027423号