当前位置:
X-MOL 学术
›
ChemBioChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Characterisation of the l-Cystine β-Lyase PatB from Phaeobacter inhibens: An Enzyme Involved in the Biosynthesis of the Marine Antibiotic Tropodithietic Acid
ChemBioChem ( IF 3.2 ) Pub Date : 2017-10-20 02:10:30 , DOI: 10.1002/cbic.201700358 Jeroen S. Dickschat 1 , Jan Rinkel 1 , Tim Klapschinski 1 , Jörn Petersen 2
ChemBioChem ( IF 3.2 ) Pub Date : 2017-10-20 02:10:30 , DOI: 10.1002/cbic.201700358 Jeroen S. Dickschat 1 , Jan Rinkel 1 , Tim Klapschinski 1 , Jörn Petersen 2
Affiliation
|
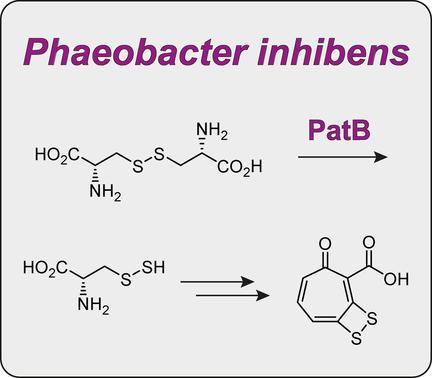
Stick in the sulfur: The l-cystine β-lyase from P. inhibens is biochemically characterised in terms of the identification of products from the natural substrate, its substrate scope and enzyme kinetics, and by site-directed mutagenesis. The obtained results, together with feeding experiments with 34S-labelled substrates, corroborate a previously suggested mechanism for sulfur incorporation into the antibiotic tropodithietic acid.
中文翻译:

苯丙氨酸杆菌的半胱氨酸β-裂合酶PatB的表征:一种涉及海洋抗生素tropodithietic酸的生物合成的酶。
棒中的硫:该升从-cystineβ裂解酶P. inhibens是生化表征在识别的产品从天然底物,它的底物范围和酶动力学方面,并通过位点定向诱变。获得的结果,再加上用34 S标记底物进行的进料实验,证实了先前提出的将硫掺入抗生素对二硫代乙酸的机理。
更新日期:2017-10-20
中文翻译:

苯丙氨酸杆菌的半胱氨酸β-裂合酶PatB的表征:一种涉及海洋抗生素tropodithietic酸的生物合成的酶。
棒中的硫:该升从-cystineβ裂解酶P. inhibens是生化表征在识别的产品从天然底物,它的底物范围和酶动力学方面,并通过位点定向诱变。获得的结果,再加上用34 S标记底物进行的进料实验,证实了先前提出的将硫掺入抗生素对二硫代乙酸的机理。



























 京公网安备 11010802027423号
京公网安备 11010802027423号