当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Aqueous-Phase Chemistry of η3-Allylpalladium(II) Complexes with Sulfonated N-Heterocyclic Carbene Ligands: Solvent Effects in the Protolysis of Pd–C Bonds and Suzuki–Miyaura Reactions
Organometallics ( IF 2.8 ) Pub Date : 2017-10-19 00:00:00 , DOI: 10.1021/acs.organomet.7b00635 Juan M. Asensio 1 , Román Andrés 1 , Pilar Gómez-Sal 1 , Ernesto de Jesús 1
Organometallics ( IF 2.8 ) Pub Date : 2017-10-19 00:00:00 , DOI: 10.1021/acs.organomet.7b00635 Juan M. Asensio 1 , Román Andrés 1 , Pilar Gómez-Sal 1 , Ernesto de Jesús 1
Affiliation
|
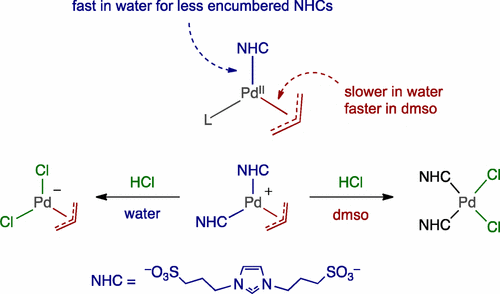
The synthesis of water-soluble η3-allyl Pd(II) complexes containing sulfonated N-heterocyclic carbene (NHC) ligands of general formula [Pd(NHC)n(η3-allyl)Cl2–n] is reported (n = 1 (1) or 2 (8)). Monocarbene complexes were obtained with the most sterically hindered NHC ligands, and biscarbenes with the less sterically hindered NHCs. The behavior of the isolated complexes in water under acidic, neutral, or alkaline conditions has been studied. The complexes are rather stable in water under neutral or alkaline conditions, although displacement of the chlorido ligand by water or hydroxide occurs under these conditions. In acidic media, Pd–NHC bonds are protolysed, and it is especially noteworthy that this protolysis occurs preferentially to that of the Pd–allyl bonds in the case of the complexes with the less-sterically hindered NHC ligands. This behavior contrasts with that observed in dimethyl sulfoxide (dmso), where the Pd–allyl bonds are selectively broken upon treatment with Brönsted acids. In addition, Pd–NHC bond breaking was promoted by the addition of a strong σ-donor ligand such as cyanide. Complex 1a is an active catalyst for the Suzuki–Miyaura cross-coupling of water-soluble aryl chlorides in neat water under moderate conditions (typically, 0.5 mol % Pd and 60 °C).
中文翻译:

的水相化学η 3 π-烯丙基(II)配合物的磺化ñ -杂环碳烯配体:溶剂效应的Pd-C键的质子迁移和铃木-宫浦反应
水溶性η的合成3含有磺化-烯丙基钯(II)络合物ñ -杂环卡宾(NHC)配体的通式[钯(NHC)Ñ(η 3 -烯丙基)氯-2- Ñ ]报道(Ñ = 1(1)或2(8))。获得了具有最受空间阻碍的NHC配体的单碳烯配合物,以及具有受较少位阻的NHC的双卡宾。已经研究了分离的配合物在酸性,中性或碱性条件下在水中的行为。该络合物在中性或碱性条件下在水中相当稳定,尽管在这些条件下会发生氯配体被水或氢氧化物置换的情况。在酸性介质中,Pd–NHC键是被质子分解的,特别值得注意的是,对于具有较少空间受阻的NHC配体的配合物,这种质子分解优先于Pd–烯丙基键的分解。此行为与在二甲基亚砜(dmso)中观察到的行为相反,在二甲基亚砜(dmso)中,用布朗斯台德酸处理后,Pd-烯丙基键选择性地断裂。此外,Pd–NHC键断裂通过加入强的σ供体配体(如氰化物)而得到促进。复杂的1a是在纯净水中在中等条件下(通常为0.5 mol%Pd和60°C)的水溶性芳基氯的Suzuki-Miyaura交叉偶联的活性催化剂。
更新日期:2017-10-20
中文翻译:

的水相化学η 3 π-烯丙基(II)配合物的磺化ñ -杂环碳烯配体:溶剂效应的Pd-C键的质子迁移和铃木-宫浦反应
水溶性η的合成3含有磺化-烯丙基钯(II)络合物ñ -杂环卡宾(NHC)配体的通式[钯(NHC)Ñ(η 3 -烯丙基)氯-2- Ñ ]报道(Ñ = 1(1)或2(8))。获得了具有最受空间阻碍的NHC配体的单碳烯配合物,以及具有受较少位阻的NHC的双卡宾。已经研究了分离的配合物在酸性,中性或碱性条件下在水中的行为。该络合物在中性或碱性条件下在水中相当稳定,尽管在这些条件下会发生氯配体被水或氢氧化物置换的情况。在酸性介质中,Pd–NHC键是被质子分解的,特别值得注意的是,对于具有较少空间受阻的NHC配体的配合物,这种质子分解优先于Pd–烯丙基键的分解。此行为与在二甲基亚砜(dmso)中观察到的行为相反,在二甲基亚砜(dmso)中,用布朗斯台德酸处理后,Pd-烯丙基键选择性地断裂。此外,Pd–NHC键断裂通过加入强的σ供体配体(如氰化物)而得到促进。复杂的1a是在纯净水中在中等条件下(通常为0.5 mol%Pd和60°C)的水溶性芳基氯的Suzuki-Miyaura交叉偶联的活性催化剂。



























 京公网安备 11010802027423号
京公网安备 11010802027423号