当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Methodology for Monobactam Diversification: Syntheses and Studies of 4-Thiomethyl Substituted β-Lactams with Activity against Gram-Negative Bacteria, Including Carbapenemase Producing Acinetobacter baumannii
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2017-10-20 00:00:00 , DOI: 10.1021/acs.jmedchem.7b01164 Serena Carosso 1 , Rui Liu 1 , Patricia A. Miller 1 , Scott J. Hecker 2 , Tomasz Glinka 2 , Marvin J. Miller 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2017-10-20 00:00:00 , DOI: 10.1021/acs.jmedchem.7b01164 Serena Carosso 1 , Rui Liu 1 , Patricia A. Miller 1 , Scott J. Hecker 2 , Tomasz Glinka 2 , Marvin J. Miller 1
Affiliation
|
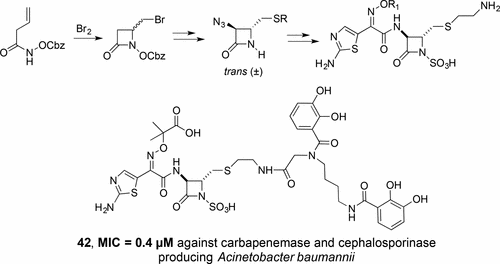
Bromine induced lactamization of vinyl acetohydroxamates facilitated syntheses of monocyclic β-lactams suitable for incorporation of a thiomethyl and extended functionality at the C(4) position. Elaboration of the resulting substituted N-hydroxy-2-azetidinones allowed incorporation of functionalized α-amino substituents appropriate for enhancement of antibiotic activity. Evaluation of antibacterial activity against a panel of Gram-positive and Gram-negative bacteria revealed structure–activity relationships (SAR) and identification of potent new monobactam antibiotics. The corresponding bis-catechol conjugate, 42, has excellent activity against Gram-negative bacteria including carbapenemase and carbacephalosporinase producing strains of Acinetobacter baumannii, which have been listed by the WHO as being of critical concern worldwide.
中文翻译:

Monobactam多元化的方法论:具有抗革兰氏阴性细菌(包括碳青霉烯酶产生鲍曼不动杆菌)活性的4-硫甲基取代的β-内酰胺的合成和研究
溴诱导的乙酰氧肟酸乙烯基酯的内酰胺化促进了适合于在C(4)位置掺入硫代甲基和扩展功能的单环β-内酰胺的合成。对所得的取代的N-羟基-2-氮杂环丁酮进行精细加工,可以引入适于增强抗生素活性的官能化α-氨基取代基。对一组革兰氏阳性和革兰氏阴性细菌的抗菌活性的评估揭示了结构-活性关系(SAR)和有效的新型单bactamtam抗生素的鉴定。相应的双邻苯二酚偶联物42对革兰氏阴性细菌(包括碳青霉烯酶和产生碳头孢菌素酶的鲍曼不动杆菌)具有优异的活性。,已被WHO列为全球关注的重点。
更新日期:2017-10-20
中文翻译:

Monobactam多元化的方法论:具有抗革兰氏阴性细菌(包括碳青霉烯酶产生鲍曼不动杆菌)活性的4-硫甲基取代的β-内酰胺的合成和研究
溴诱导的乙酰氧肟酸乙烯基酯的内酰胺化促进了适合于在C(4)位置掺入硫代甲基和扩展功能的单环β-内酰胺的合成。对所得的取代的N-羟基-2-氮杂环丁酮进行精细加工,可以引入适于增强抗生素活性的官能化α-氨基取代基。对一组革兰氏阳性和革兰氏阴性细菌的抗菌活性的评估揭示了结构-活性关系(SAR)和有效的新型单bactamtam抗生素的鉴定。相应的双邻苯二酚偶联物42对革兰氏阴性细菌(包括碳青霉烯酶和产生碳头孢菌素酶的鲍曼不动杆菌)具有优异的活性。,已被WHO列为全球关注的重点。











































 京公网安备 11010802027423号
京公网安备 11010802027423号