当前位置:
X-MOL 学术
›
ACS Comb. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Parallel Synthesis of 1H-Pyrazolo[3,4-d]pyrimidines via Condensation of N-Pyrazolylamides and Nitriles
ACS Combinatorial Science Pub Date : 2017-10-20 00:00:00 , DOI: 10.1021/acscombsci.7b00116 Akshay A. Shah 1 , Lois K. Chenard 1 , Joseph W. Tucker 1 , Christopher J. Helal 1
ACS Combinatorial Science Pub Date : 2017-10-20 00:00:00 , DOI: 10.1021/acscombsci.7b00116 Akshay A. Shah 1 , Lois K. Chenard 1 , Joseph W. Tucker 1 , Christopher J. Helal 1
Affiliation
|
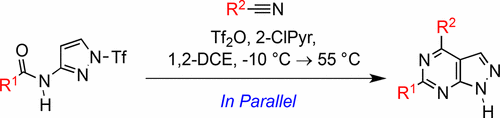
A novel parallel medicinal chemistry (PMC)-enabled synthesis of 1H-pyrazolo[3,4-d]pyrimidines employing condensation of easily accessible N-pyrazolylamides and nitriles has been developed. The presented studies describe singleton and library enablements that allowed rapid generation of molecular diversity to examine C4 and C6 vectors. This chemistry enabled access to challenging alkyl substituents, expanding the overall chemical space beyond that available via typical C(sp2)–C(sp2) coupling and SNAr transformations. Furthermore, monomer group interconversions allowing the use of larger and more diverse amides and carboxylic acids as precursors to nitriles are discussed.
中文翻译:

N-吡唑基酰亚胺和腈的缩合反应并行合成1 H-吡唑并[3,4- d ]嘧啶
已开发出一种新颖的平行药物化学(PMC)合成方法,可利用易于获得的N-吡唑基酰胺与腈的缩合反应合成1 H-吡唑并[3,4- d ]嘧啶。提出的研究描述了单例和库使能,其允许快速产生分子多样性来检查C4和C6载体。这种化学性质使人们能够获得具有挑战性的烷基取代基,从而扩大了整个化学空间,使其超过了典型的C(sp 2)–C(sp 2)偶联和S N Ar转化所能提供的空间。此外,讨论了允许使用更大和更多样化的酰胺和羧酸作为腈前体的单体基团互变。
更新日期:2017-10-20
中文翻译:

N-吡唑基酰亚胺和腈的缩合反应并行合成1 H-吡唑并[3,4- d ]嘧啶
已开发出一种新颖的平行药物化学(PMC)合成方法,可利用易于获得的N-吡唑基酰胺与腈的缩合反应合成1 H-吡唑并[3,4- d ]嘧啶。提出的研究描述了单例和库使能,其允许快速产生分子多样性来检查C4和C6载体。这种化学性质使人们能够获得具有挑战性的烷基取代基,从而扩大了整个化学空间,使其超过了典型的C(sp 2)–C(sp 2)偶联和S N Ar转化所能提供的空间。此外,讨论了允许使用更大和更多样化的酰胺和羧酸作为腈前体的单体基团互变。











































 京公网安备 11010802027423号
京公网安备 11010802027423号