PLoS Pathogens ( IF 5.5 ) Pub Date : 2017-10-19 , DOI: 10.1371/journal.ppat.1006631 Tiffani Alvey Jones , Diane Z. Hernandez , Zoë C. Wong , Anica M. Wandler , Karen Guillemin
|
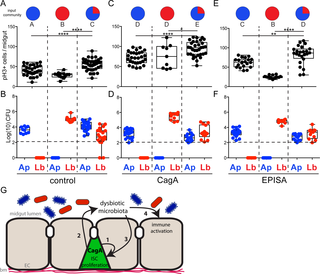
Gut microbiota facilitate many aspects of human health and development, but dysbiotic microbiota can promote hyperplasia and inflammation and contribute to human diseases such as cancer. Human patients infected with the gastric cancer-causing bacterium Helicobacter pylori have altered microbiota; however, whether dysbiosis contributes to disease in this case is unknown. Many H. pylori human disease phenotypes are associated with a potent virulence protein, CagA, which is translocated into host epithelial cells where it alters cell polarity and manipulates host-signaling pathways to promote disease. We hypothesized that CagA alone could contribute to H. pylori pathogenesis by inducing microbial dysbiosis that promotes disease. Here we use a transgenic Drosophila model of CagA expression to genetically disentangle the effects of the virulence protein CagA from that of H. pylori infection. We found that expression of CagA within Drosophila intestinal stem cells promotes excess cell proliferation and is sufficient to alter host microbiota. Rearing CagA transgenic flies germ-free revealed that the dysbiotic microbiota contributes to cell proliferation phenotypes and also elicits expression of innate immune components, Diptericin and Duox. Further investigations revealed interspecies interactions are required for this dysbiotic CagA-dependent microbiota to promote proliferation in CagA transgenic and healthy control Drosophila. Our model establishes that CagA can alter gut microbiota and exacerbate cell proliferation and immune phenotypes previously attributed to H. pylori infection. This work provides valuable new insights into the mechanisms by which interactions between a specific virulence factor and the resident microbiota can contribute to the development and progression of disease.
中文翻译:

细菌毒力因子CagA诱导微生物营养不良,导致果蝇肠道中的上皮细胞过度增殖
肠道菌群可促进人类健康和发展的许多方面,但不良生物菌群可促进增生和炎症,并助长人类疾病,例如癌症。感染了引起胃癌的幽门螺杆菌的人类患者的菌群发生了改变。然而,在这种情况下,营养不良是否会导致疾病尚不清楚。许多^ h。幽门螺杆菌的人类疾病表型与有效的毒力蛋白CagA相关,CagA易位到宿主上皮细胞中,在那里它改变细胞极性并操纵宿主信号传导途径来促进疾病。我们推测,CagA的单独有助于^ h。幽门螺杆菌通过诱导促进疾病的微生物营养不良而致病。在这里,我们使用CagA表达的转基因果蝇模型从基因上分离了毒蛋白CagA与H的作用。幽门螺杆菌感染。我们发现果蝇中CagA的表达肠道干细胞可促进细胞过度增殖,足以改变宿主菌群。饲养的CagA转基因果蝇经无菌处理后发现,该不良生物微生物群有助于细胞增殖表型,并引起先天性免疫成分,双歧杆菌素和Duox的表达。进一步的研究表明,这种不良生物的依赖CagA的微生物群需要种间相互作用,以促进CagA转基因和健康对照果蝇中的增殖。我们的模型建立了CagA可以改变肠道菌群并加剧以前归因于H的细胞增殖和免疫表型。幽门螺杆菌感染。这项工作为特定毒力因子与微生物群落之间的相互作用可能有助于疾病发展和发展的机理提供了有价值的新见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号