当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure–Activity Relationship, Pharmacological Characterization, and Molecular Modeling of Noncompetitive Inhibitors of the Betaine/γ-Aminobutyric Acid Transporter 1 (BGT1)
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2017-10-19 00:00:00 , DOI: 10.1021/acs.jmedchem.7b00924 Lars Jørgensen 1 , Anas Al-Khawaja 1 , Stefanie Kickinger 2 , Stine B. Vogensen 1 , Jonas Skovgaard-Petersen 1 , Emil Rosenthal 1 , Nrupa Borkar 1 , Rebekka Löffler 1 , Karsten K. Madsen 1 , Hans Bräuner-Osborne 1 , Arne Schousboe 1 , Gerhard F. Ecker 2 , Petrine Wellendorph 1 , Rasmus P. Clausen 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2017-10-19 00:00:00 , DOI: 10.1021/acs.jmedchem.7b00924 Lars Jørgensen 1 , Anas Al-Khawaja 1 , Stefanie Kickinger 2 , Stine B. Vogensen 1 , Jonas Skovgaard-Petersen 1 , Emil Rosenthal 1 , Nrupa Borkar 1 , Rebekka Löffler 1 , Karsten K. Madsen 1 , Hans Bräuner-Osborne 1 , Arne Schousboe 1 , Gerhard F. Ecker 2 , Petrine Wellendorph 1 , Rasmus P. Clausen 1
Affiliation
|
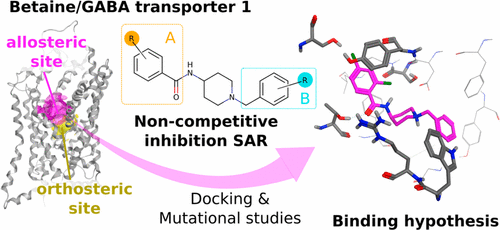
N-(1-Benzyl-4-piperidinyl)-2,4-dichlorobenzamide 5 (BPDBA) is a noncompetitive inhibitor of the betaine/GABA transporter 1 (BGT1). We here report the synthesis and structure–activity relationship of 71 analogues. We identify 26m as a more soluble 2,4-Cl substituted 3-pyridine analogue with retained BGT1 activity and an improved off-target profile compared to 5. We performed radioligand-based uptake studies at chimeric constructs between BGT1 and GAT3, experiments with site-directed mutated transporters, and computational docking in a BGT1 homology model based on the newly determined X-ray crystal structure of the human serotonin transporter (hSERT). On the basis of these experiments, we propose a binding mode involving residues within TM10 in an allosteric site in BGT1 that corresponds to the allosteric binding pocket revealed by the hSERT crystal structure. Our study provides first insights into a proposed allosteric binding pocket in BGT1, which accommodates the binding site for a series of novel noncompetitive inhibitors.
中文翻译:

甜菜碱/γ-氨基丁酸转运蛋白1(BGT1)的非竞争性抑制剂的结构-活性关系,药理特性和分子模型
N-(1-苄基-4-哌啶基)-2,4-二氯苯甲酰胺5(BPDBA)是甜菜碱/ GABA转运蛋白1(BGT1)的非竞争性抑制剂。我们在这里报告了71个类似物的合成及其构效关系。我们确定26m为具有2种保留的BGT1活性和比5更好的脱靶轮廓的2,4-Cl取代的3,4-吡啶取代的可溶性类似物。我们对BGT1和GAT3之间的嵌合构建体进行了基于放射性配体的摄取研究,对定点突变的转运蛋白进行了实验,并基于新确定的人类血清素转运蛋白(hSERT)的X射线晶体结构,将其对接到BGT1同源模型中。在这些实验的基础上,我们提出了一种结合模式,该模式涉及BGT1变构位点TM10中的残基,该残基对应于hSERT晶体结构揭示的变构结合口袋。我们的研究提供了对BGT1中提议的变构结合口袋的初步见解,该口袋可容纳一系列新型非竞争性抑制剂的结合位点。
更新日期:2017-10-19
中文翻译:

甜菜碱/γ-氨基丁酸转运蛋白1(BGT1)的非竞争性抑制剂的结构-活性关系,药理特性和分子模型
N-(1-苄基-4-哌啶基)-2,4-二氯苯甲酰胺5(BPDBA)是甜菜碱/ GABA转运蛋白1(BGT1)的非竞争性抑制剂。我们在这里报告了71个类似物的合成及其构效关系。我们确定26m为具有2种保留的BGT1活性和比5更好的脱靶轮廓的2,4-Cl取代的3,4-吡啶取代的可溶性类似物。我们对BGT1和GAT3之间的嵌合构建体进行了基于放射性配体的摄取研究,对定点突变的转运蛋白进行了实验,并基于新确定的人类血清素转运蛋白(hSERT)的X射线晶体结构,将其对接到BGT1同源模型中。在这些实验的基础上,我们提出了一种结合模式,该模式涉及BGT1变构位点TM10中的残基,该残基对应于hSERT晶体结构揭示的变构结合口袋。我们的研究提供了对BGT1中提议的变构结合口袋的初步见解,该口袋可容纳一系列新型非竞争性抑制剂的结合位点。











































 京公网安备 11010802027423号
京公网安备 11010802027423号