当前位置:
X-MOL 学术
›
Cell Chem. Bio.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure-Guided Development of a Potent and Selective Non-covalent Active-Site Inhibitor of USP7
Cell Chemical Biology ( IF 6.6 ) Pub Date : 2017-10-19 , DOI: 10.1016/j.chembiol.2017.09.003 Ilaria Lamberto , Xiaoxi Liu , Hyuk-Soo Seo , Nathan J. Schauer , Roxana E. Iacob , Wanyi Hu , Deepika Das , Tatiana Mikhailova , Ellen L. Weisberg , John R. Engen , Kenneth C. Anderson , Dharminder Chauhan , Sirano Dhe-Paganon , Sara J. Buhrlage
Cell Chemical Biology ( IF 6.6 ) Pub Date : 2017-10-19 , DOI: 10.1016/j.chembiol.2017.09.003 Ilaria Lamberto , Xiaoxi Liu , Hyuk-Soo Seo , Nathan J. Schauer , Roxana E. Iacob , Wanyi Hu , Deepika Das , Tatiana Mikhailova , Ellen L. Weisberg , John R. Engen , Kenneth C. Anderson , Dharminder Chauhan , Sirano Dhe-Paganon , Sara J. Buhrlage
|
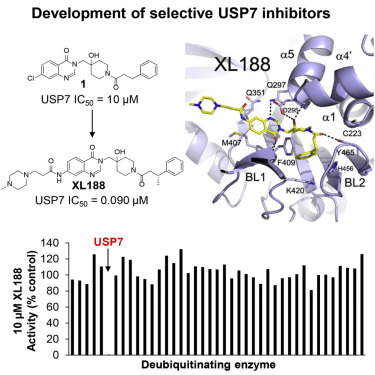
Deubiquitinating enzymes (DUBs) have garnered significant attention as drug targets in the last 5–10 years. The excitement stems in large part from the powerful ability of DUB inhibitors to promote degradation of oncogenic proteins, especially proteins that are challenging to directly target but which are stabilized by DUB family members. Highly optimized and well-characterized DUB inhibitors have thus become highly sought after tools. Most reported DUB inhibitors, however, are polypharmacological agents possessing weak (micromolar) potency toward their primary target, limiting their utility in target validation and mechanism studies. Due to a lack of high-resolution DUB⋅small-molecule ligand complex structures, no structure-guided optimization efforts have been reported for a mammalian DUB. Here, we report a small-molecule⋅ubiquitin-specific protease (USP) family DUB co-structure and rapid design of potent and selective inhibitors of USP7 guided by the structure. Interestingly, the compounds are non-covalent active-site inhibitors.
中文翻译:

USP7强效和选择性非共价活性位点抑制剂的结构指导开发。
在过去的5-10年中,去泛素化酶(DUBs)作为药物靶标受到了广泛关注。兴奋的主要原因在于DUB抑制剂具有促进致癌蛋白降解的强大能力,尤其是对直接靶向具有挑战性但被DUB家族成员稳定的蛋白。因此,高度优化和特征明确的DUB抑制剂已成为人们追捧的工具。然而,大多数报道的DUB抑制剂是多药制剂,其对主要靶标的效价较弱(微摩尔),从而限制了其在靶标验证和机理研究中的效用。由于缺乏高分辨率的DUB·小分子配体复合物结构,因此尚未报道针对哺乳动物DUB的结构指导的优化工作。这里,我们报道了小分子泛素特异性蛋白酶(USP)家族DUB的共结构,并根据该结构快速设计了USP7的有效和选择性抑制剂。有趣的是,这些化合物是非共价活性位点抑制剂。
更新日期:2017-12-21
中文翻译:

USP7强效和选择性非共价活性位点抑制剂的结构指导开发。
在过去的5-10年中,去泛素化酶(DUBs)作为药物靶标受到了广泛关注。兴奋的主要原因在于DUB抑制剂具有促进致癌蛋白降解的强大能力,尤其是对直接靶向具有挑战性但被DUB家族成员稳定的蛋白。因此,高度优化和特征明确的DUB抑制剂已成为人们追捧的工具。然而,大多数报道的DUB抑制剂是多药制剂,其对主要靶标的效价较弱(微摩尔),从而限制了其在靶标验证和机理研究中的效用。由于缺乏高分辨率的DUB·小分子配体复合物结构,因此尚未报道针对哺乳动物DUB的结构指导的优化工作。这里,我们报道了小分子泛素特异性蛋白酶(USP)家族DUB的共结构,并根据该结构快速设计了USP7的有效和选择性抑制剂。有趣的是,这些化合物是非共价活性位点抑制剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号