Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
STING Senses Microbial Viability to Orchestrate Stress-Mediated Autophagy of the Endoplasmic Reticulum.
Cell ( IF 45.5 ) Pub Date : 2017-Nov-02 , DOI: 10.1016/j.cell.2017.09.034 Julien Moretti , Soumit Roy , Dominique Bozec , Jennifer Martinez , Jessica R. Chapman , Beatrix Ueberheide , Dudley W. Lamming , Zhijian J. Chen , Tiffany Horng , Garabet Yeretssian , Douglas R. Green , J. Magarian Blander
Cell ( IF 45.5 ) Pub Date : 2017-Nov-02 , DOI: 10.1016/j.cell.2017.09.034 Julien Moretti , Soumit Roy , Dominique Bozec , Jennifer Martinez , Jessica R. Chapman , Beatrix Ueberheide , Dudley W. Lamming , Zhijian J. Chen , Tiffany Horng , Garabet Yeretssian , Douglas R. Green , J. Magarian Blander
|
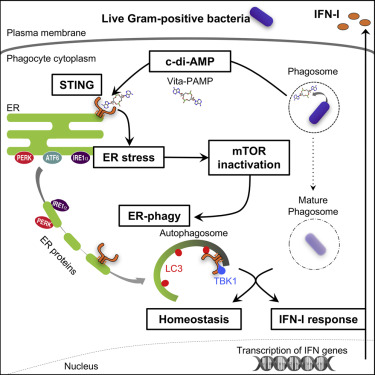
Constitutive cell-autonomous immunity in metazoans predates interferon-inducible immunity and comprises primordial innate defense. Phagocytes mobilize interferon-inducible responses upon engagement of well-characterized signaling pathways by pathogen-associated molecular patterns (PAMPs). The signals controlling deployment of constitutive cell-autonomous responses during infection have remained elusive. Vita-PAMPs denote microbial viability, signaling the danger of cellular exploitation by intracellular pathogens. We show that cyclic-di-adenosine monophosphate in live Gram-positive bacteria is a vita-PAMP, engaging the innate sensor stimulator of interferon genes (STING) to mediate endoplasmic reticulum (ER) stress. Subsequent inactivation of the mechanistic target of rapamycin mobilizes autophagy, which sequesters stressed ER membranes, resolves ER stress, and curtails phagocyte death. This vita-PAMP-induced ER-phagy additionally orchestrates an interferon response by localizing ER-resident STING to autophagosomes. Our findings identify stress-mediated ER-phagy as a cell-autonomous response mobilized by STING-dependent sensing of a specific vita-PAMP and elucidate how innate receptors engage multilayered homeostatic mechanisms to promote immunity and survival after infection.
中文翻译:

STING感知微生物生存能力,以协调内质网的应激介导的自噬。
后生动物的本构细胞自主免疫早于干扰素诱导的免疫,并包括原始的先天防御。吞噬细胞通过病原体相关的分子模式(PAMPs)参与了特征明确的信号通路,从而动员了干扰素诱导的应答。控制感染期间本构细胞自主反应的部署的信号仍然难以捉摸。维生素-PAMPs表示微生物的生存能力,表明细胞内病原体对细胞进行剥削的危险。我们显示活革兰氏阳性细菌中的环二腺苷单磷酸酯是一种vita-PAMP,参与干扰素基因(STING)的先天性传感器刺激物来介导内质网(ER)应激。随后,雷帕霉素机理性靶点的失活动员了自噬,这会隔离应激的ER膜,解决内质网应激,并减少吞噬细胞死亡。这种vita-PAMP诱导的ER噬菌体还可以通过将ER驻留STING定位于自噬体来协调干扰素应答。我们的发现将应激介导的ER吞噬识别为一种细胞自主性反应,通过对特定vita-PAMP的STING依赖性传感而动员,并阐明了先天受体如何参与多层稳态机制以提高感染后的免疫力和存活率。
更新日期:2017-10-19
中文翻译:

STING感知微生物生存能力,以协调内质网的应激介导的自噬。
后生动物的本构细胞自主免疫早于干扰素诱导的免疫,并包括原始的先天防御。吞噬细胞通过病原体相关的分子模式(PAMPs)参与了特征明确的信号通路,从而动员了干扰素诱导的应答。控制感染期间本构细胞自主反应的部署的信号仍然难以捉摸。维生素-PAMPs表示微生物的生存能力,表明细胞内病原体对细胞进行剥削的危险。我们显示活革兰氏阳性细菌中的环二腺苷单磷酸酯是一种vita-PAMP,参与干扰素基因(STING)的先天性传感器刺激物来介导内质网(ER)应激。随后,雷帕霉素机理性靶点的失活动员了自噬,这会隔离应激的ER膜,解决内质网应激,并减少吞噬细胞死亡。这种vita-PAMP诱导的ER噬菌体还可以通过将ER驻留STING定位于自噬体来协调干扰素应答。我们的发现将应激介导的ER吞噬识别为一种细胞自主性反应,通过对特定vita-PAMP的STING依赖性传感而动员,并阐明了先天受体如何参与多层稳态机制以提高感染后的免疫力和存活率。











































 京公网安备 11010802027423号
京公网安备 11010802027423号