当前位置:
X-MOL 学术
›
JAMA Psychiatry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effectiveness of Injectable Extended-Release Naltrexone vs Daily Buprenorphine-Naloxone for Opioid Dependence: A Randomized Clinical Noninferiority Trial.
JAMA Psychiatry ( IF 22.5 ) Pub Date : 2017-12-01 , DOI: 10.1001/jamapsychiatry.2017.3206 Lars Tanum 1, 2 , Kristin Klemmetsby Solli 1 , Zill-E-Huma Latif 2 , Jurate Šaltyte Benth 3, 4 , Arild Opheim 5, 6 , Kamni Sharma-Haase 1, 7 , Peter Krajci 8 , Nikolaj Kunøe 1
JAMA Psychiatry ( IF 22.5 ) Pub Date : 2017-12-01 , DOI: 10.1001/jamapsychiatry.2017.3206 Lars Tanum 1, 2 , Kristin Klemmetsby Solli 1 , Zill-E-Huma Latif 2 , Jurate Šaltyte Benth 3, 4 , Arild Opheim 5, 6 , Kamni Sharma-Haase 1, 7 , Peter Krajci 8 , Nikolaj Kunøe 1
Affiliation
|
Importance
To date, extended-release naltrexone hydrochloride has not previously been compared directly with opioid medication treatment (OMT), currently the most commonly prescribed treatment for opioid dependence.
Objective
To determine whether treatment with extended-release naltrexone will be as effective as daily buprenorphine hydrochloride with naloxone hydrochloride in maintaining abstinence from heroin and other illicit substances in newly detoxified individuals.
Design, Setting and Participants
A 12-week, multicenter, outpatient, open-label randomized clinical trial was conducted at 5 urban addiction clinics in Norway between November 1, 2012, and December 23, 2015; the last follow-up was performed on October 23, 2015. A total of 232 adult opioid-dependent (per DSM-IV criteria) individuals were recruited from outpatient addiction clinics and detoxification units and assessed for eligibility. Intention-to-treat analyses of efficacy end points were performed with all randomized participants.
Interventions
Randomization to either daily oral flexible dose buprenorphine-naloxone, 4 to 24 mg/d, or extended-release naltrexone hydrochloride, 380 mg, administered intramuscularly every fourth week for 12 weeks.
Main Outcomes and Measures
Primary end points (protocol) were the randomized clinical trial completion rate, the proportion of opioid-negative urine drug tests, and number of days of use of heroin and other illicit opioids. Secondary end points included number of days of use of other illicit substances. Safety was assessed by adverse event reporting.
Results
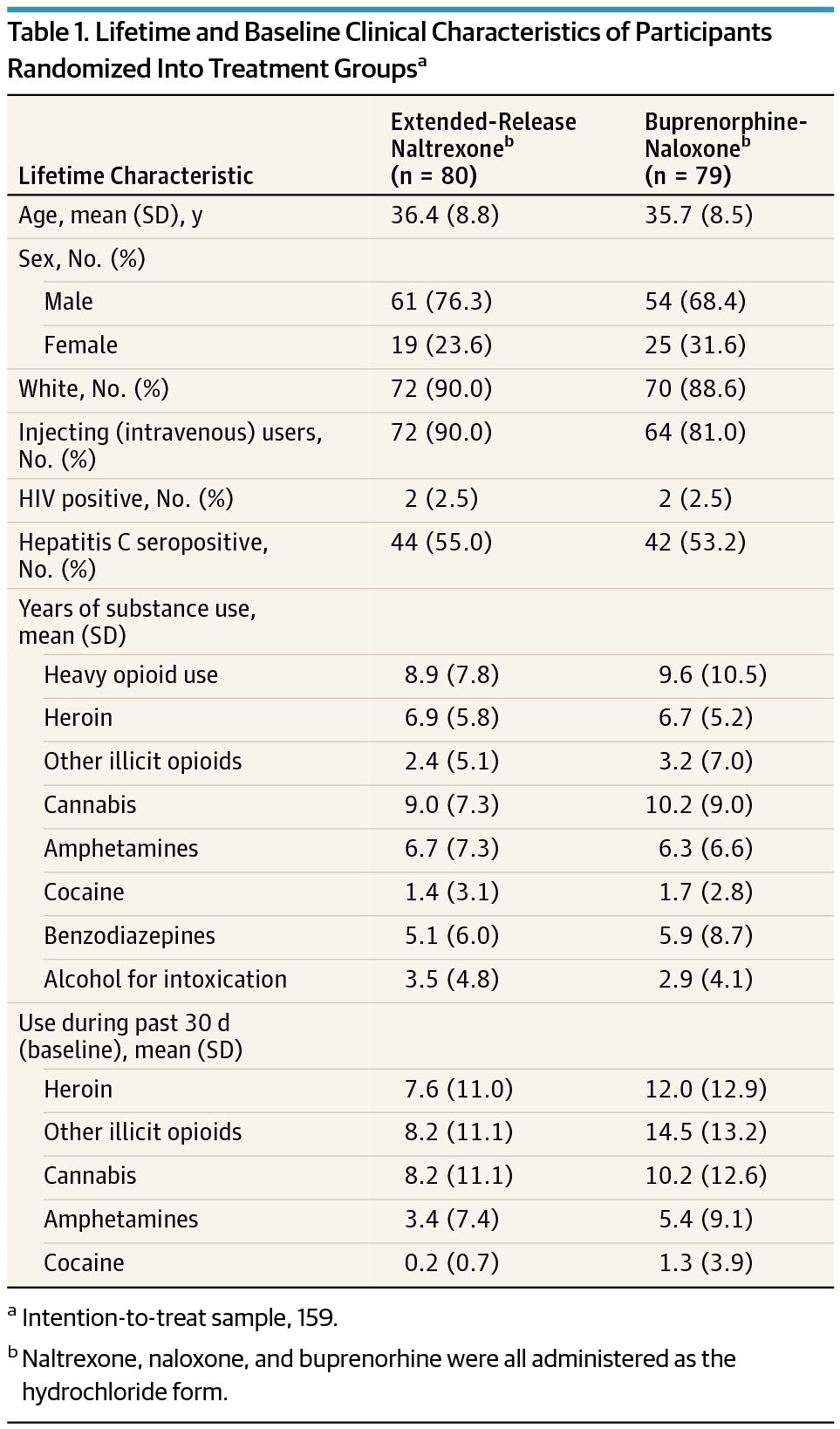
Of 159 participants, mean (SD) age was 36 (8.6) years and 44 (27.7%) were women. Eighty individuals were randomized to extended-release naltrexone and 79 to buprenorphine-naloxone; 105 (66.0%) completed the trial. Retention in the extended-release naltrexone group was noninferior to the buprenorphine-naloxone group (difference, -0.1; with 95% CI, -0.2 to 0.1; P = .04), with mean (SD) time of 69.3 (25.9) and 63.7 (29.9) days, correspondingly (P = .33, log-rank test). Treatment with extended-release naltrexone showed noninferiority to buprenorphine-naloxone on group proportion of total number of opioid-negative urine drug tests (mean [SD], 0.9 [0.3] and 0.8 [0.4], respectively, difference, 0.1 with 95% CI, -0.04 to 0.2; P < .001) and use of heroin (mean difference, -3.2 with 95% CI, -4.9 to -1.5; P < .001) and other illicit opioids (mean difference, -2.7 with 95% CI, -4.6 to -0.9; P < .001). Superiority analysis showed significantly lower use of heroin and other illicit opioids in the extended-release naltrexone group. No significant differences were found between the treatment groups regarding most other illicit substance use.
Conclusions and Relevance
Extended-release naltrexone was as effective as buprenorphine-naloxone in maintaining short-term abstinence from heroin and other illicit substances and should be considered as a treatment option for opioid-dependent individuals.
Trial Registration
clinicaltrials.gov Identifier: NCT01717963.
中文翻译:

注射缓释纳曲酮与每日丁丙诺啡-纳洛酮对阿片类药物依赖的有效性:随机临床非劣效性试验。
重要性 迄今为止,盐酸纳曲酮缓释剂尚未与阿片类药物治疗 (OMT) 进行直接比较,阿片类药物治疗是目前阿片类药物依赖最常用的治疗方法。目的 确定缓释纳曲酮治疗在维持刚戒毒个体戒除海洛因和其他非法物质方面是否与每日盐酸丁丙诺啡联合盐酸纳洛酮治疗一样有效。设计、设置和参与者 2012年11月1日至2015年12月23日期间,在挪威5个城市成瘾诊所进行了一项为期12周、多中心、门诊、开放标签的随机临床试验;最后一次随访于 2015 年 10 月 23 日进行。从门诊成瘾诊所和戒毒单位招募了总共 232 名成人阿片类药物依赖者(根据 DSM-IV 标准),并评估其资格。对所有随机参与者进行了疗效终点的意向治疗分析。干预措施 随机分为每日口服灵活剂量丁丙诺啡-纳洛酮,4 至 24 mg/d,或缓释盐酸纳曲酮,380 mg,每第四周肌肉注射一次,持续 12 周。主要结果和措施 主要终点(方案)是随机临床试验完成率、阿片类药物尿液检测呈阴性的比例以及使用海洛因和其他非法阿片类药物的天数。次要终点包括使用其他非法物质的天数。通过不良事件报告评估安全性。结果 159 名参与者中,平均 (SD) 年龄为 36 (8.6) 岁,其中 44 名 (27.7%) 为女性。 80 名受试者被随机分配至缓释纳曲酮组,79 名受试者被随机分配至丁丙诺啡-纳洛酮组; 105(66。0%)完成了试验。缓释纳曲酮组的保留不劣于丁丙诺啡-纳洛酮组(差异,-0.1;95% CI,-0.2 至 0.1;P = .04),平均 (SD) 时间为 69.3 (25.9),相应地为 63.7 (29.9) 天(P = .33,对数秩检验)。缓释纳曲酮治疗显示,在阿片类药物阴性尿液药物检测总数的组比例方面,不劣于丁丙诺啡-纳洛酮(平均值 [SD] 分别为 0.9 [0.3] 和 0.8 [0.4],差异为 0.1,95% CI ,-0.04 至 0.2;P < .001)和使用海洛因(平均差异,-3.2,95% CI,-4.9 至 -1.5;P < .001)和其他非法阿片类药物(平均差异,-2.7,95% CI,-4.6 至 -0.9;P < .001)。优越性分析显示,缓释纳曲酮组海洛因和其他非法阿片类药物的使用率显着降低。在大多数其他非法物质使用方面,治疗组之间没有发现显着差异。结论和相关性 缓释纳曲酮在维持短期戒除海洛因和其他非法物质方面与丁丙诺啡-纳洛酮一样有效,应被视为阿片类药物依赖个体的治疗选择。试验注册 ClinicalTrials.gov 标识符:NCT01717963。
更新日期:2017-12-06
中文翻译:

注射缓释纳曲酮与每日丁丙诺啡-纳洛酮对阿片类药物依赖的有效性:随机临床非劣效性试验。
重要性 迄今为止,盐酸纳曲酮缓释剂尚未与阿片类药物治疗 (OMT) 进行直接比较,阿片类药物治疗是目前阿片类药物依赖最常用的治疗方法。目的 确定缓释纳曲酮治疗在维持刚戒毒个体戒除海洛因和其他非法物质方面是否与每日盐酸丁丙诺啡联合盐酸纳洛酮治疗一样有效。设计、设置和参与者 2012年11月1日至2015年12月23日期间,在挪威5个城市成瘾诊所进行了一项为期12周、多中心、门诊、开放标签的随机临床试验;最后一次随访于 2015 年 10 月 23 日进行。从门诊成瘾诊所和戒毒单位招募了总共 232 名成人阿片类药物依赖者(根据 DSM-IV 标准),并评估其资格。对所有随机参与者进行了疗效终点的意向治疗分析。干预措施 随机分为每日口服灵活剂量丁丙诺啡-纳洛酮,4 至 24 mg/d,或缓释盐酸纳曲酮,380 mg,每第四周肌肉注射一次,持续 12 周。主要结果和措施 主要终点(方案)是随机临床试验完成率、阿片类药物尿液检测呈阴性的比例以及使用海洛因和其他非法阿片类药物的天数。次要终点包括使用其他非法物质的天数。通过不良事件报告评估安全性。结果 159 名参与者中,平均 (SD) 年龄为 36 (8.6) 岁,其中 44 名 (27.7%) 为女性。 80 名受试者被随机分配至缓释纳曲酮组,79 名受试者被随机分配至丁丙诺啡-纳洛酮组; 105(66。0%)完成了试验。缓释纳曲酮组的保留不劣于丁丙诺啡-纳洛酮组(差异,-0.1;95% CI,-0.2 至 0.1;P = .04),平均 (SD) 时间为 69.3 (25.9),相应地为 63.7 (29.9) 天(P = .33,对数秩检验)。缓释纳曲酮治疗显示,在阿片类药物阴性尿液药物检测总数的组比例方面,不劣于丁丙诺啡-纳洛酮(平均值 [SD] 分别为 0.9 [0.3] 和 0.8 [0.4],差异为 0.1,95% CI ,-0.04 至 0.2;P < .001)和使用海洛因(平均差异,-3.2,95% CI,-4.9 至 -1.5;P < .001)和其他非法阿片类药物(平均差异,-2.7,95% CI,-4.6 至 -0.9;P < .001)。优越性分析显示,缓释纳曲酮组海洛因和其他非法阿片类药物的使用率显着降低。在大多数其他非法物质使用方面,治疗组之间没有发现显着差异。结论和相关性 缓释纳曲酮在维持短期戒除海洛因和其他非法物质方面与丁丙诺啡-纳洛酮一样有效,应被视为阿片类药物依赖个体的治疗选择。试验注册 ClinicalTrials.gov 标识符:NCT01717963。











































 京公网安备 11010802027423号
京公网安备 11010802027423号