Journal of Controlled Release ( IF 10.5 ) Pub Date : 2017-10-18 , DOI: 10.1016/j.jconrel.2017.10.029 Binghua Wang , Yongxia Zhai , Jinjin Shi , Luyang Zhuang , Wei Liu , Huijuan Zhang , Hongling Zhang , Zhenzhong Zhang
|
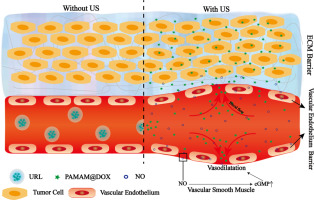
Tumor vascular endothelium and extracellular matrix (ECM) as the major barriers of anticancer nanomedicine greatly limited the anticancer efficacy of treatment, but few strategies were available to overcome them simultaneously. Thus, herein a strategy was presented to utilize reversible vasodilatation effect of nitric oxide (NO) and size-controlled characteristic of ultrasound responsive liposome (URL) to construct a non-destructive nanomedicine, which was able to cross both obstacles simultaneously. In this work, URL was built as a carrier via forming a gas layer between lipid bilayer to encapsulate small particles [email protected] (PD, ~ 10 nm) and NO donation-nitrosoglutathione (GSNO). Under ultrasound (US) stimulation, GSNO fastly generated NO that acting on tumor vascular smooth muscle, resulting in tumor vascular vasodilatation, meanwhile the URL lipid bilayer was destroyed, leading to release sharply of small nanoparticles PD. Combination vasodilatory effect of NO and size-controlled characteristic of URL allowed vast drugs to extravasate through endothelial gap and penetrate into tumor deep. Upon different types of cancers vary greatly in vascular structure, two distinctly different tumor, MCF-7 human breast carcinoma and MiaPaCa-2 human pancreatic carcinoma, were chosen to test the anticancer efficacy of URL. As a result, URL-based nanosystem was significantly more effective than the conventional liposome (CL) in tumor treatment, particularly in much less leaky MiaPaCa-2 tumor treatment (tumor therapeutic efficiency of URL/PD/GSNO + US increased by 32.5% and 56.5% compared to CL/DOX in MCF-7 and MiaPaCa-2 tumor treatment). This study offers a new method to enhance tumor drug accumulation along with minimal toxicity for future clinical cancer treatments.
中文翻译:

通过无损尺寸控制的纳米药物同时克服肿瘤血管内皮和细胞外基质障碍
肿瘤血管内皮和细胞外基质(ECM)作为抗癌纳米药物的主要障碍极大地限制了其治疗的抗癌效果,但很少有能同时克服它们的策略。因此,本文提出了利用一氧化氮(NO)的可逆性血管舒张作用和超声响应脂质体(URL)的尺寸控制特征来构建能够同时克服两个障碍的非破坏性纳米药物的策略。在这项工作中,URL是通过在脂质双层之间形成气体层以封装小颗粒(受电子邮件保护)(PD,〜10 nm)和NO供体硝基谷胱甘肽(GSNO)的载体而构建的。在超声(US)刺激下,GSNO迅速生成作用于肿瘤血管平滑肌的NO,导致肿瘤血管舒张,同时,URL脂质双层被破坏,导致纳米颗粒PD急剧释放。NO与URL的大小控制特征的结合血管舒张作用使大量药物通过内皮间隙渗出并深入肿瘤。当不同类型的癌症的血管结构发生巨大变化时,选择两种截然不同的肿瘤MCF-7人乳腺癌和MiaPaCa-2人胰腺癌来测试URL的抗癌效果。结果,基于URL的纳米系统在肿瘤治疗中比传统脂质体(CL)显着更有效,特别是在泄漏少的MiaPaCa-2肿瘤治疗中(URL / PD / GSNO + US的肿瘤治疗效率提高了32.5%,与MC / C-7和MiaPaCa-2肿瘤治疗中的CL / DOX相比,降低了56.5%。











































 京公网安备 11010802027423号
京公网安备 11010802027423号