当前位置:
X-MOL 学术
›
ACS Synth. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rational Design of an Anticalin-Type Sugar-Binding Protein Using a Genetically Encoded Boronate Side Chain
ACS Synthetic Biology ( IF 3.7 ) Pub Date : 2017-10-18 00:00:00 , DOI: 10.1021/acssynbio.7b00199 Selvakumar Edwardraja 1 , Andreas Eichinger 1 , Ina Theobald 1 , Carina Andrea Sommer 1 , Andreas J. Reichert 1 , Arne Skerra 1
ACS Synthetic Biology ( IF 3.7 ) Pub Date : 2017-10-18 00:00:00 , DOI: 10.1021/acssynbio.7b00199 Selvakumar Edwardraja 1 , Andreas Eichinger 1 , Ina Theobald 1 , Carina Andrea Sommer 1 , Andreas J. Reichert 1 , Arne Skerra 1
Affiliation
|
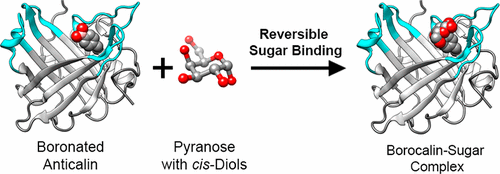
The molecular recognition of carbohydrates plays a fundamental role in many biological processes. However, the development of carbohydrate-binding reagents for biomedical research and use poses a challenge due to the generally poor affinity of proteins toward sugars in aqueous solution. Here, we describe the effective molecular recognition of pyranose monosaccharides (in particular, galactose and mannose) by a rationally designed protein receptor based on the human lipocalin scaffold (Anticalin). Complexation relies on reversible covalent cis-diol boronate diester formation with a genetically encoded l-boronophenylalanine (Bpa) residue which was incorporated as a non-natural amino acid at a sterically permissive position in the ligand pocket of the Anticalin, as confirmed by X-ray crystallography. Compared with the metal-ion and/or avidity-dependent oligovalent lectins that prevail in nature, our approach offers a novel and promising route to generate tight sugar-binding reagents both as research reagents and for biomedical applications.
中文翻译:

使用遗传编码的硼酸盐侧链合理设计Anticalin型糖结合蛋白
碳水化合物的分子识别在许多生物学过程中都起着基本作用。然而,由于蛋白质对水溶液中糖类的亲和力通常较差,因此用于生物医学研究和使用的碳水化合物结合剂的开发提出了挑战。在这里,我们描述了通过合理设计的基于人脂蛋白支架(Anticalin)的蛋白质受体,对吡喃糖单糖(特别是半乳糖和甘露糖)进行有效的分子识别。络合依赖于可逆的共价顺-二醇二酯硼酸酯形成用遗传编码的升X射线晶体学证实,β-硼烷苯丙氨酸(Bpa)残基作为非天然氨基酸掺入在Anticalin配体口袋的空间允许位置。与自然界中普遍存在的依赖金属离子和/或亲和力的寡价凝集素相比,我们的方法提供了一种新颖且有希望的途径来生成紧密的糖结合剂,既可作为研究试剂,也可用于生物医学应用。
更新日期:2017-10-18
中文翻译:

使用遗传编码的硼酸盐侧链合理设计Anticalin型糖结合蛋白
碳水化合物的分子识别在许多生物学过程中都起着基本作用。然而,由于蛋白质对水溶液中糖类的亲和力通常较差,因此用于生物医学研究和使用的碳水化合物结合剂的开发提出了挑战。在这里,我们描述了通过合理设计的基于人脂蛋白支架(Anticalin)的蛋白质受体,对吡喃糖单糖(特别是半乳糖和甘露糖)进行有效的分子识别。络合依赖于可逆的共价顺-二醇二酯硼酸酯形成用遗传编码的升X射线晶体学证实,β-硼烷苯丙氨酸(Bpa)残基作为非天然氨基酸掺入在Anticalin配体口袋的空间允许位置。与自然界中普遍存在的依赖金属离子和/或亲和力的寡价凝集素相比,我们的方法提供了一种新颖且有希望的途径来生成紧密的糖结合剂,既可作为研究试剂,也可用于生物医学应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号