当前位置:
X-MOL 学术
›
Corros. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An experimental and theoretical approach towards understanding the inhibitive behavior of a nitrile substituted coumarin compound as an effective acidic media inhibitor
Corrosion Science ( IF 7.4 ) Pub Date : 2018-04-01 , DOI: 10.1016/j.corsci.2017.10.004 Alper Fitoz , Hasan Nazır , Mehtap Özgür (nee Yakut) , Emel Emregül , Kaan C. Emregül
Corrosion Science ( IF 7.4 ) Pub Date : 2018-04-01 , DOI: 10.1016/j.corsci.2017.10.004 Alper Fitoz , Hasan Nazır , Mehtap Özgür (nee Yakut) , Emel Emregül , Kaan C. Emregül
|
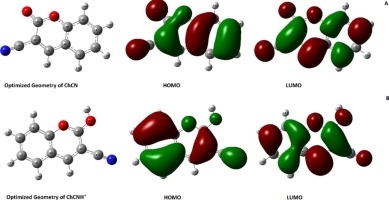
Abstract The inhibiting ability of 2H-chromen-2-one (Ch) or coumarin and its corresponding nitrile substituted compound 2-Oxo-2H-chromen-3-carbonitrile (ChCN), has been studied using electrochemical techniques, SEM and theoretical calculation methods The Langmuir adsorption isotherm model best described the adsorption characteristics of the compounds under study. Scanning electron microscopy studies revealed the formation of a protective inhibitor layer on the mild steel surface. Quantum chemical parameters obtained using the density functional theory (DFT) supported the experimental results. Substitution of a CN group was seen to increase the inhibitor efficiency up to 98%.
中文翻译:

了解腈取代香豆素化合物作为有效酸性介质抑制剂的抑制行为的实验和理论方法
摘要 使用电化学技术、SEM 和理论计算方法研究了 2H-chromen-2-one (Ch) 或香豆素及其相应的腈取代化合物 2-Oxo-2H-chromen-3-carbonitrile (ChCN) 的抑制能力。 Langmuir 吸附等温线模型最好地描述了所研究化合物的吸附特性。扫描电子显微镜研究表明在低碳钢表面形成了保护性抑制剂层。使用密度泛函理论 (DFT) 获得的量子化学参数支持实验结果。发现 CN 基团的取代可将抑制剂效率提高至 98%。
更新日期:2018-04-01
中文翻译:

了解腈取代香豆素化合物作为有效酸性介质抑制剂的抑制行为的实验和理论方法
摘要 使用电化学技术、SEM 和理论计算方法研究了 2H-chromen-2-one (Ch) 或香豆素及其相应的腈取代化合物 2-Oxo-2H-chromen-3-carbonitrile (ChCN) 的抑制能力。 Langmuir 吸附等温线模型最好地描述了所研究化合物的吸附特性。扫描电子显微镜研究表明在低碳钢表面形成了保护性抑制剂层。使用密度泛函理论 (DFT) 获得的量子化学参数支持实验结果。发现 CN 基团的取代可将抑制剂效率提高至 98%。







































 京公网安备 11010802027423号
京公网安备 11010802027423号