Journal of Fluorine Chemistry ( IF 1.7 ) Pub Date : 2017-10-16 , DOI: 10.1016/j.jfluchem.2017.10.007 Mahmoud Hamdoush , Ivan A. Skvortsov , Maksim S. Mikhailov , Georgy Pakhomov , Pavel A. Stuzhin
|
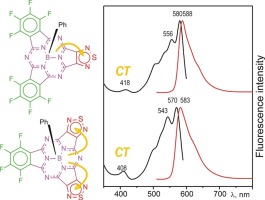
Two low-symmetry analogues of perfluorinated subphthalocyanine bearing 1,2,5-thiadiazole ring(s) instead one or two benzene rings were synthesized by cocyclotrimerization of tetrafluorophthalonitrile and 1,2,5-thiadiazolo-3,4-dicarbonitrile in the presence of dichlorophenylboron(III) in chlorobenzene. New subporphyrazines have being characterized by mass-spectrometry, NMR spectroscopy (1H, 11B, 19F), electronic absorption and emission spectra. Reduction of the symmetry of the suborphyrazine π-chromophore lifts degeneracy of the LUMO and leads to a split Q-band in the visible region (∼540–550 and ∼570–580 nm). New absorption band due to charge transfer between the subporphyrazine core and fused π-electron deficient 1,2,5-thiadiazole rings appears at 390–420 nm.
中文翻译:

含有稠合的1,2,5-噻二唑片段的全氟亚酞菁类似物
通过四氟邻苯二甲腈和1,2,5-噻二唑-3,4-二碳腈的共环三聚反应,合成了带有1,2,5-噻二唑环而不是一个或两个苯环的全氟化亚酞菁的两个低对称类似物。氯苯中的二氯苯基硼(III)。新型亚卟啉已经通过质谱,NMR光谱进行了表征(1 H,11 B,19F),电子吸收和发射光谱。降低甲氧杂卟啉π发色团的对称性会提高LUMO的简并性,并导致可见光区(〜540–550和570〜580 nm)中的Q波段分裂。由于亚卟啉核心与稠合的π电子缺陷的1,2,5-噻二唑环之间的电荷转移,新的吸收带出现在390–420 nm处。











































 京公网安备 11010802027423号
京公网安备 11010802027423号