当前位置:
X-MOL 学术
›
J. Fluorine Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Highly regio- and stereoselective 1,3-dipolar cycloaddition of stabilised azomethine ylides to 3,3,3-trihalogeno-1-nitropropenes: Synthesis of trihalomethylated spiroindenepyrroli(zi)dines
Journal of Fluorine Chemistry ( IF 1.7 ) Pub Date : 2017-10-16 , DOI: 10.1016/j.jfluchem.2017.10.005 Alexey Yu. Barkov , Nikolay S. Zimnitskiy , Igor B. Kutyashev , Vladislav Yu. Korotaev , Vladimir S. Moshkin , Vyacheslav Ya. Sosnovskikh
中文翻译:

高度偶氮和立体选择性的1,3-偶极环化的稳定的甲亚胺烷基化物加成至3,3,3-三卤代-1-硝基丙烯:三卤代甲基化的螺茚并吡咯并(zi)二烯的合成
更新日期:2017-10-16
Journal of Fluorine Chemistry ( IF 1.7 ) Pub Date : 2017-10-16 , DOI: 10.1016/j.jfluchem.2017.10.005 Alexey Yu. Barkov , Nikolay S. Zimnitskiy , Igor B. Kutyashev , Vladislav Yu. Korotaev , Vladimir S. Moshkin , Vyacheslav Ya. Sosnovskikh
|
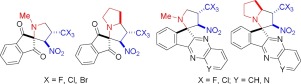
Reactions of (E)-3,3,3-trihalogeno-1-nitropropenes with stabilised azomethine ylides derived from ninhydrin and indenoquinoxalinones on the one hand, and sarcosine and proline on the other, proceed regio- and diastereoselectively to give a number of trihalomethylated spiroindenepyrrolidines and spiroindenepyrrolizidines in good yields.
中文翻译:

高度偶氮和立体选择性的1,3-偶极环化的稳定的甲亚胺烷基化物加成至3,3,3-三卤代-1-硝基丙烯:三卤代甲基化的螺茚并吡咯并(zi)二烯的合成
(E)-3,3,3-三卤代-1-硝基丙烯与由茚三酮和茚并喹喔啉酮衍生的稳定的甲亚胺基化物,另一方面与肌氨酸和脯氨酸的反应,在区域和非对映异构体上选择性进行,得到许多三卤代甲基化螺茚并吡咯烷和螺螺茚并吡咯烷的产率高。











































 京公网安备 11010802027423号
京公网安备 11010802027423号