Catalysis Today ( IF 5.2 ) Pub Date : 2017-10-16 , DOI: 10.1016/j.cattod.2017.10.014 Kalidas B. Rasal , Ganapati D. Yadav
|
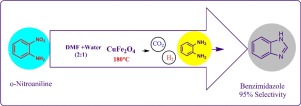
One pot synthesis of benzimidazole from o-nitroaniline was achieved by using CuFe2O4 as a catalyst. It comprises the reduction of o-nitroaniline followed by cyclization, without using an external H2 source. The thermal decomposition of DMF in situ generates CO, which undergo water gas shift reaction (WGSR) in the presence of CuFe2O4 to produce hydrogen. It reduces −NO2 (nitroaniline) to −NH2 (o-phenylenediamine, OPD). The further cyclisation of OPD to benzimidazole was done by using DMF as a C1 source, in the presence of magnetically separable CuFe2O4 as catalyst. This is the first example of its kind being reported here. The catalyst was prepared by a simple hydrothermal method, with an environmentally benign starting material. CuFe2O4 is cheap and reusable having very low toxicity. This is an economical synthetic protocol for benzimidazole from o-nitroaniline with 100% conversion in 12 h with 97.5% selectivity. A variety of o-nitroaniline substrates were studied using the protocol with excellent conversion and selectivity in each case.
中文翻译:

在CuFe 2 O 4为催化剂的条件下,以DMF为多任务试剂单锅合成苯并咪唑
通过使用CuFe 2 O 4作为催化剂,由邻硝基苯胺一锅合成苯并咪唑。它包括在不使用外部H 2源的情况下还原邻硝基苯胺,然后环化。DMF的热分解原位生成CO,在CuFe 2 O 4的存在下,CO发生水煤气变换反应(WGSR)以产生氢气。它将-NO 2(硝基苯胺)还原为-NH 2(邻苯二胺,OPD)。在磁性可分离的CuFe 2 O存在下,通过使用DMF作为C1源,将OPD进一步环化为苯并咪唑4作为催化剂。这是这里报道的同类的第一个例子。通过简单的水热法,使用对环境无害的起始原料制备催化剂。CuFe 2 O 4便宜且可重复使用,且毒性极低。这是从邻硝基苯胺合成苯并咪唑的经济方法,在12小时内100%转化,选择性为97.5%。使用该方案研究了各种邻硝基苯胺底物,在每种情况下均具有出色的转化率和选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号