Journal of Controlled Release ( IF 10.5 ) Pub Date : 2017-10-16 , DOI: 10.1016/j.jconrel.2017.10.017 Beat M. Jucker , Hasan Alsaid , Mary Rambo , Stephen C. Lenhard , Bao Hoang , Fang Xie , M. Reid Groseclose , Stephen Castellino , Valeriu Damian , Gary Bowers , Manish Gupta
|
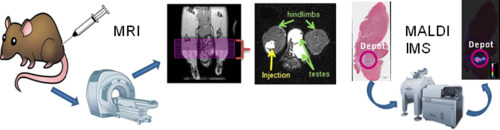
Long-Acting Parenterals (LAPs) have been used in the clinic to provide sustained therapeutic drug levels at a target site, and thereby reducing the frequency of dosing required. In an effort to understand the factors associated with long-acting cabotegravir (GSK1265744 LAP) pharmacokinetic variability, the current study was designed to investigate the temporal relationship between intramuscular (IM) or subcutaneous (SC) drug depot morphology and distribution kinetics with plasma pharmacokinetics. Therefore, a multi-modal molecular imaging (MRI & MALDI IMS) approach was employed to examine the temporal GSK1265744 LAP biodistribution in rat following either IM or SC administration. Serial MRI was performed immediately post drug administration, and then at day 1 (24 h post), 2, 3, 4, 7, and 14. In a separate cohort of rats, an MRI contrast agent, Feraheme® (USPIO), was administered 2 days post IM drug injection in order to investigate the potential involvement of macrophages trafficking to the GSK1265744 LAP and Vehicle depot sites. The GSK1265744 LAP depot volume increased rapidly by day 2 in the IM injected rats (~ 3–7 fold) compared with a ~ 1 fold increase in the SC injected rats. In addition, the USPIO contrast agent labeled macrophages were shown to be present in the depot region of the GSK1265744 LAP injected gastrocnemius while the Vehicle injected gastrocnemius appeared to show reduced uptake. Matrix-assisted laser desorption ionization (MALDI) imaging mass spectrometry (IMS) of muscle and abdominal tissue sections identified the drug content primarily within the depot. Co-registration of the GSK1265744 ion images with immunohistochemical images established that the drug was taken up by macrophages associated with the depot. Linear regression analysis demonstrated that the drug depot characteristics including volume, surface area, and perimeter assessed by MRI at day 2 correlated with early time point plasma drug concentrations.
In summary, a multimodal molecular imaging approach was used to identify the drug depot location and volumetric/physiologic changes in both IM and SC locations following GSK1265744 LAP administration. The IM depot volume increased rapidly to a maximum volume at 2 days post-GSK1265744 LAP administration, while the Vehicle depot did not suggesting that the active drug substance and/or related particle was a key driver for drug depot evolution. The depot expansion was associated with an increase in macrophage infiltration and edema in and around the depot region and was correlated to plasma drug concentration at early time points (0–4 days). Consequently, molecular imaging approaches may be used in patients to help understand the biodistribution of GSK1265744 LAP and its associated pharmacokinetics.
中文翻译:

多峰成像方法检查长效肠胃外制剂Cabotegravir(GSK1265744)在大鼠中的生物分布动力学
长效肠胃外药物(LAP)已在临床中用于在目标部位提供持续的治疗药物水平,从而减少了所需的给药频率。为了了解与长效Cabotegravir(GSK1265744 LAP)药代动力学变异性相关的因素,本研究旨在研究肌内(IM)或皮下(SC)药库形态与血浆药代动力学之间的分布动力学之间的时间关系。因此,采用多模态分子成像(MRI和MALDI IMS)方法来检查IM或SC给药后大鼠的颞GSK1265744 LAP生物分布。药物给药后立即进行系列MRI,然后在第1天(给药后24小时),2、3、4、7和14。在另一组大鼠中,使用MRI造影剂,IM药物注射后2天服用Feraheme®(USPIO),以调查巨噬细胞向GSK1265744 LAP和车辆停放站运送的潜在可能性。在第2天,IM注射大鼠的GSK1265744 LAP贮藏库容量迅速增加(约3至7倍),而SC注射大鼠则增加了约1倍。此外,USPIO造影剂标记的巨噬细胞显示存在于GSK1265744 LAP注射的腓肠肌的储库区,而媒介物注射的腓肠肌似乎显示出摄取减少。肌肉和腹部组织切片的基质辅助激光解吸电离(MALDI)成像质谱(IMS)识别出主要在仓库内的药物含量。GSK1265744离子图像与免疫组织化学图像的共配准建立了该药物被与该储库相关的巨噬细胞吸收的结果。线性回归分析表明,第2天通过MRI评估的药物储库特征(包括体积,表面积和周长)与早期血浆药物浓度相关。
总而言之,在服用GSK1265744 LAP后,使用多峰分子成像方法来鉴定药物存放处的位置以及IM和SC位置的体积/生理变化。在GSK1265744 LAP给药后2天,IM库的体积迅速增加到最大体积,而Vehicle库并未表明活性药物和/或相关颗粒是药物库发展的关键驱动力。贮库的扩张与贮库区域内和周围的巨噬细胞浸润和水肿的增加有关,并且与早期时间点(0–4天)的血浆药物浓度相关。因此,分子成像方法可用于患者,以帮助了解GSK1265744 LAP的生物分布及其相关的药代动力学。











































 京公网安备 11010802027423号
京公网安备 11010802027423号