Applied Catalysis A: General ( IF 5.5 ) Pub Date : 2017-10-16 , DOI: 10.1016/j.apcata.2017.10.009 I.Tyrone Ghampson , Roberto Canales , Néstor Escalona
|
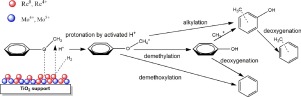
A well-characterized Re-MoOx/TiO2 catalyst was used to investigate the reaction sequence involved during the hydrodeoxygenation of anisole in a batch reactor by varying the initial anisole concentration in the reactant mixture (0.182–0.382 mol L−1 corresponding to 2.6–5.4 wt.%), the reaction temperature (250–325 °C) and hydrogen pressure (30–60 bar). The effects of these process variables on product selectivity, calculated at 10% anisole conversion, and reaction rate, estimated from the initial slope of anisole conversion vs. time, were discussed. The initial reaction rate was observed to increase to a maximum at anisole concentration of 0.282 mol L−1 and then decrease at higher concentrations. Mathematical modeling of the proposed reaction network revealed that the decreased activity at higher anisole concentration is related to diminished ability to cleave CO bonds on phenol and cresols, probably due to increased surface coverage by the reactant on the oxophilic sites on the catalyst which limits access to surface reactive hydrogen for transformation. In regards to the influence of reaction temperature, high temperature favors the formation of cresols at the expense of benzene which was confirmed by the estimated apparent activation energy of the different pathways. On the other hand, the observed strong dependence of benzene/phenol ratio on H2 pressure is attributed to increased availability of surface reactive hydrogen surface to aid in oxygen removal. Results from this study and the literature indicates that C
O bond breaking is preceded by either protonation of oxygen on anisole or by partial hydrogenation of the aromatic ring to lower the C
O scission barrier. Analysis of the conversion of intermediate compounds confirmed the reaction sequence and preferences during anisole HDO on Re-MoOx/TiO2 catalyst.
中文翻译:

Re-MoO x / TiO 2催化剂上苯甲醚加氢脱氧的研究
表征良好的Re-MoO x / TiO 2催化剂用于通过改变反应混合物中苯甲醚的初始浓度(0.182–0.382 mol L -1对应于2.6)来研究分批反应器中苯甲醚加氢脱氧过程中涉及的反应顺序。–5.4 wt。%),反应温度(250–325°C)和氢气压力(30–60 bar)。讨论了这些工艺变量对以10%苯甲醚转化率计算的产物选择性和根据苯甲醚转化率与时间的初始斜率估算的反应速率的影响。观察到初始反应速率在苯甲醚浓度为0.282 mol L -1时增加到最大值然后在更高的浓度下降低。拟议中的反应网络的数学模型表明,在较高的苯甲醚浓度下,活性降低与裂解苯酚和甲酚上的C O键的能力降低有关,这可能是由于反应物在催化剂的亲氧位点上增加了表面覆盖率,从而限制了进入表面活性氢进行转化。关于反应温度的影响,高温有利于甲酚的形成,但以苯为代价,这由不同途径的估计表观活化能所证实。另一方面,观察到苯/苯酚比率对H 2的强烈依赖性压力归因于增加了表面活性氢表面的可用性,以帮助除氧。这项研究和文献的结果表明,
在苯甲醚上的氧质子化或芳香环的部分氢化以降低C
O的分裂势垒之前,C O的键断裂。中间体化合物转化的分析证实了苯甲醚HDO在Re-MoO x / TiO 2催化剂上的反应顺序和偏好。



























 京公网安备 11010802027423号
京公网安备 11010802027423号