当前位置:
X-MOL 学术
›
React. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Telescoped continuous flow generation of a library of highly substituted 3-thio-1,2,4-triazoles
Reaction Chemistry & Engineering ( IF 3.9 ) Pub Date : 2017-10-04 00:00:00 , DOI: 10.1039/c7re00125h Mariana C. F. C. B. Damião 1, 2, 3, 4 , Renan Galaverna 1, 2, 3, 4 , Alan P. Kozikowski 5, 6, 7, 8 , James Eubanks 9, 10, 11, 12 , Julio C. Pastre 1, 2, 3, 4
Reaction Chemistry & Engineering ( IF 3.9 ) Pub Date : 2017-10-04 00:00:00 , DOI: 10.1039/c7re00125h Mariana C. F. C. B. Damião 1, 2, 3, 4 , Renan Galaverna 1, 2, 3, 4 , Alan P. Kozikowski 5, 6, 7, 8 , James Eubanks 9, 10, 11, 12 , Julio C. Pastre 1, 2, 3, 4
Affiliation
|
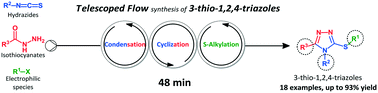
We report herein the successful application of continuous flow micro reactors to prepare important building blocks based on the 3-thio-1,2,4-triazole core. A telescoped continuous flow process was developed based on the condensation of hydrazides and isothiocyanates to deliver an in situ stream of a thiosemicarbazide, which subsequently was cyclized under basic conditions. The obtained 1,2,4-triazole-3-thiol was further alkylated with benzyl/alkyl halides. In addition, we evaluated the scope of heterocycle formation and alkylation using different hydrazides, isothiocyanates, and aryl/alkyl chlorides, bromides and iodides. We were able to synthesize a small library of 18 compounds in 48 minutes of residence time for each synthesis, and in moderate to excellent yields, in a telescoped fashion. The fully integrated synthesis flow platform enables the fast generation of compound libraries, reducing the time consumed in preliminary stages of a drug discovery process.
中文翻译:

伸缩连续流生成高度取代的3-thio-1,2,4-三唑库
我们在此报告了连续流微型反应器在基于3-硫基1,2,4-三唑核心制备重要构件的成功应用。甲伸缩连续流动过程是基于酰肼的缩合开发和异硫氰酸酯以递送的原位硫代氨基脲的物流,随后在碱性条件下环化。将获得的1,2,4-三唑-3-硫醇进一步用苄基/烷基卤化物烷基化。此外,我们评估了使用不同的酰肼,异硫氰酸酯和芳基/烷基氯化物,溴化物和碘化物形成杂环和烷基化的范围。对于每次合成,我们能够在48分钟的停留时间内合成出18种化合物的小型文库,并且以望远镜的方式合成了中等至优异的收率。完全集成的合成流程平台能够快速生成化合物库,从而减少了药物发现过程初步阶段所消耗的时间。
更新日期:2017-10-16
中文翻译:

伸缩连续流生成高度取代的3-thio-1,2,4-三唑库
我们在此报告了连续流微型反应器在基于3-硫基1,2,4-三唑核心制备重要构件的成功应用。甲伸缩连续流动过程是基于酰肼的缩合开发和异硫氰酸酯以递送的原位硫代氨基脲的物流,随后在碱性条件下环化。将获得的1,2,4-三唑-3-硫醇进一步用苄基/烷基卤化物烷基化。此外,我们评估了使用不同的酰肼,异硫氰酸酯和芳基/烷基氯化物,溴化物和碘化物形成杂环和烷基化的范围。对于每次合成,我们能够在48分钟的停留时间内合成出18种化合物的小型文库,并且以望远镜的方式合成了中等至优异的收率。完全集成的合成流程平台能够快速生成化合物库,从而减少了药物发现过程初步阶段所消耗的时间。



























 京公网安备 11010802027423号
京公网安备 11010802027423号