Journal of Controlled Release ( IF 10.8 ) Pub Date : 2017-10-14 , DOI: 10.1016/j.jconrel.2017.10.005 Lalit K. Vora , Ryan F. Donnelly , Eneko Larrañeta , Patricia González-Vázquez , Raghu Raj Singh Thakur , Pradeep R. Vavia
|
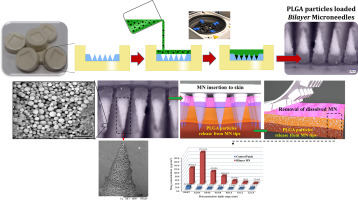
Polymeric microneedle (MN) arrays continue to receive growing attention due to their ability to bypass the skin's stratum corneum barrier in a minimally-invasive fashion and achieve enhanced transdermal drug delivery and “targeted” intradermal vaccine administration. In this research work, we fabricated biodegradable bilayer MN arrays containing nano - microparticles for targeted and sustained intradermal drug delivery. For this study, model drug (vitamin D3, VD3)-loaded PLGA nano- and microparticles (NMP) were prepared by a single emulsion solvent evaporation method with 72.8% encapsulation of VD3. The prepared NMP were directly mixed 20% w/v poly(vinyl pyrrolidone) (PVP) gel, with the mixture filled into laser engineered micromoulds by high-speed centrifugation (30 min) to concentrate NMP into MN shafts. The particle size of PLGA NMP ranged from 300 nm to 3.5 μm and they retained their particle size after moulding of bilayer MN arrays. The relatively wide particle size distribution of PLGA NMP was shown to be important in producing a compact structure in bilayer conical, as well as pyramidal, MN, as confirmed by scanning electron microscopy. The drug release profile from PLGA NMP was tri-phasic, being sustained over 5 days. The height of bilayer MN arrays was influenced by the weight ratio of NMP and 20% w/v PVP. Good mechanical and insertion profiles (into a skin simulant and excised neonatal porcine skin) were confirmed by texture analysis and optical coherence tomography, respectively. Ex vivo intradermal neonatal porcine skin penetration of VD3 NMP from bilayer MN was quantitatively analysed after cryostatic skin sectioning, with 74.2 ± 9.18% of VD3 loading delivered intradermally. The two-stage novel processing strategy developed here provides a simple and easy method for localising particulate delivery systems into dissolving MN. Such systems may serve as promising means for controlled transdermal delivery and targeted intradermal administration.
中文翻译:

具有浓缩PLGA纳米微粒的新型双层溶解微针阵列,用于靶向皮内递送:概念验证
聚合物微针(MN)阵列由于能够以微创的方式绕过皮肤的角质层屏障并实现增强的透皮药物输送和“靶向”皮内疫苗施用能力而继续受到越来越多的关注。在这项研究工作中,我们制造了包含纳米微粒的可生物降解的双层MN阵列,用于靶向和持续的皮内药物输送。对于本研究,通过单乳液溶剂蒸发法制备了载有模型药物(维生素D 3,VD 3)的PLGA纳米和微粒(NMP),其中VD 3的包封率为72.8%。。将制备的NMP直接混合20%w / v聚乙烯吡咯烷酮(PVP)凝胶,并通过高速离心(30分钟)将混合物填充到激光加工的微型模具中,以将NMP浓缩到MN轴中。PLGA NMP的粒径范围为300 nm至3.5μm,在成型双层MN阵列后,它们仍保持其粒径。扫描电子显微镜证实,PLGA NMP的相对较宽的粒径分布对于在双层圆锥形和锥形MN中产生紧凑的结构非常重要。PLGA NMP的药物释放曲线是三相的,持续5天以上。双层MN阵列的高度受NMP和20%w / v PVP的重量比影响。冷冻皮肤切片后,对双层MN的VD 3 NMP的离体皮内新生儿猪皮肤渗透性进行了定量分析,皮内递送了74.2±9.18%的VD 3负荷。此处开发的两阶段新颖处理策略为将微粒输送系统定位到溶解MN中提供了一种简单的方法。这样的系统可以用作控制透皮递送和靶向皮内施用的有前途的手段。



























 京公网安备 11010802027423号
京公网安备 11010802027423号