当前位置:
X-MOL 学术
›
ACS Cent. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Methane Monofunctionalization by an Electrogenerated High-Valent Pd Intermediate
ACS Central Science ( IF 12.7 ) Pub Date : 2017-10-12 00:00:00 , DOI: 10.1021/acscentsci.7b00342 Matthew E O'Reilly 1 , R Soyoung Kim 1 , Seokjoon Oh 1 , Yogesh Surendranath 1
ACS Central Science ( IF 12.7 ) Pub Date : 2017-10-12 00:00:00 , DOI: 10.1021/acscentsci.7b00342 Matthew E O'Reilly 1 , R Soyoung Kim 1 , Seokjoon Oh 1 , Yogesh Surendranath 1
Affiliation
|
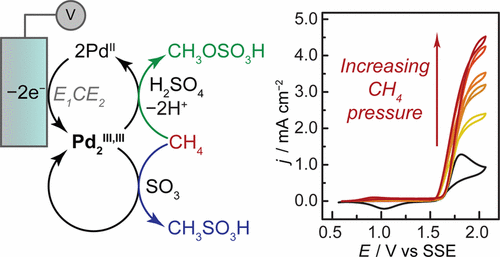
Electrophilic high-valent metal ions are potent intermediates for the catalytic functionalization of methane, but in many cases, their high redox potentials make these intermediates difficult or impossible to access using mild stoichiometric oxidants derived from O2. Herein, we establish electrochemical oxidation as a versatile new strategy for accessing high-valent methane monofunctionalization catalysts. We provide evidence for the electrochemical oxidation of simple PdSO4 in concentrated sulfuric acid electrolytes to generate a putative Pd2III,III species in an all-oxidic ligand field. This electrogenerated high-valent Pd complex rapidly activates methane with a low barrier of 25.9 (±2.6) kcal/mol, generating methanol precursors methyl bisulfate (CH3OSO3H) and methanesulfonic acid (CH3SO3H) via concurrent faradaic and nonfaradaic reaction pathways. This work enables new electrochemical approaches for promoting rapid methane monofunctionalization.
中文翻译:

电化学高价钯中间体催化甲烷单官能化
亲电高价金属离子是甲烷催化功能化的有效中间体,但在许多情况下,它们的高氧化还原电位使得这些中间体很难或不可能使用源自O 2的温和化学计量氧化剂获得。在此,我们将电化学氧化确立为一种获取高价甲烷单官能化催化剂的通用新策略。我们提供了简单 PdSO 4在浓硫酸电解质中电化学氧化以在全氧化配体场中生成假定的 Pd 2 III,III物质的证据。这种电生成的高价 Pd络合物以 25.9 (± 2.6 ) kcal/mol 的低势垒快速活化甲烷,通过并发法拉第和非法拉第反应途径。这项工作使得新的电化学方法能够促进甲烷的快速单官能化。
更新日期:2017-10-13
中文翻译:

电化学高价钯中间体催化甲烷单官能化
亲电高价金属离子是甲烷催化功能化的有效中间体,但在许多情况下,它们的高氧化还原电位使得这些中间体很难或不可能使用源自O 2的温和化学计量氧化剂获得。在此,我们将电化学氧化确立为一种获取高价甲烷单官能化催化剂的通用新策略。我们提供了简单 PdSO 4在浓硫酸电解质中电化学氧化以在全氧化配体场中生成假定的 Pd 2 III,III物质的证据。这种电生成的高价 Pd络合物以 25.9 (± 2.6 ) kcal/mol 的低势垒快速活化甲烷,通过并发法拉第和非法拉第反应途径。这项工作使得新的电化学方法能够促进甲烷的快速单官能化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号