PLoS Pathogens ( IF 5.5 ) Pub Date : 2017-10-12 , DOI: 10.1371/journal.ppat.1006613 Rachel S Leibman 1, 2 , Max W Richardson 1, 2 , Christoph T Ellebrecht 3 , Colby R Maldini 1, 2 , Joshua A Glover 4 , Anthony J Secreto 4 , Irina Kulikovskaya 2 , Simon F Lacey 2 , Sarah R Akkina 1 , Yanjie Yi 4 , Farida Shaheen 4 , Jianbin Wang 5 , Keith A Dufendach 1, 2 , Michael C Holmes 5 , Ronald G Collman 4 , Aimee S Payne 3 , James L Riley 1, 2
|
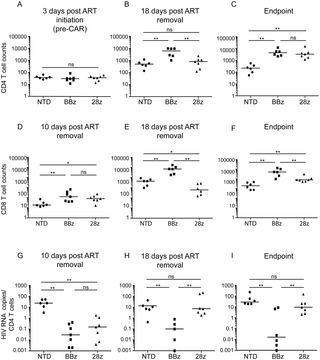
HIV is adept at avoiding naturally generated T cell responses; therefore, there is a need to develop HIV-specific T cells with greater potency for use in HIV cure strategies. Starting with a CD4-based chimeric antigen receptor (CAR) that was previously used without toxicity in clinical trials, we optimized the vector backbone, promoter, HIV targeting moiety, and transmembrane and signaling domains to determine which components augmented the ability of T cells to control HIV replication. This re-engineered CAR was at least 50-fold more potent in vitro at controlling HIV replication than the original CD4 CAR, or a TCR-based approach, and substantially better than broadly neutralizing antibody-based CARs. A humanized mouse model of HIV infection demonstrated that T cells expressing optimized CARs were superior at expanding in response to antigen, protecting CD4 T cells from infection, and reducing viral loads compared to T cells expressing the original, clinical trial CAR. Moreover, in a humanized mouse model of HIV treatment, CD4 CAR T cells containing the 4-1BB costimulatory domain controlled HIV spread after ART removal better than analogous CAR T cells containing the CD28 costimulatory domain. Together, these data indicate that potent HIV-specific T cells can be generated using improved CAR design and that CAR T cells could be important components of an HIV cure strategy.
中文翻译:

表达重新设计的基于 CD4 的嵌合抗原受体的 CD8 T 细胞介导的 HIV-1 复制的超生理控制
HIV 善于避免自然产生的 T 细胞反应;因此,需要开发具有更大效力的 HIV 特异性 T 细胞,用于 HIV 治疗策略。从之前在临床试验中使用的无毒性的基于 CD4 的嵌合抗原受体 (CAR) 开始,我们优化了载体主链、启动子、HIV 靶向部分以及跨膜和信号传导域,以确定哪些组件增强了 T 细胞的能力控制艾滋病病毒复制。这种重新设计的 CAR 在体外控制 HIV 复制方面比原始 CD4 CAR 或基于 TCR 的方法有效至少 50 倍,并且比基于广泛中和抗体的 CAR 明显更好。HIV 感染的人源化小鼠模型表明,与表达原始临床试验 CAR 的 T 细胞相比,表达优化 CAR 的 T 细胞在响应抗原而扩增、保护 CD4 T 细胞免受感染以及减少病毒载量方面表现出色。此外,在 HIV 治疗的人源化小鼠模型中,含有 4-1BB 共刺激结构域的 CD4 CAR T 细胞在 ART 去除后比含有 CD28 共刺激结构域的类似 CAR T 细胞更好地控制了 HIV 传播。总之,这些数据表明,使用改进的 CAR 设计可以产生有效的 HIV 特异性 T 细胞,并且 CAR T 细胞可能是 HIV 治疗策略的重要组成部分。











































 京公网安备 11010802027423号
京公网安备 11010802027423号