当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure–Activity Relationship Studies with Tetrahydroquinoline Analogs as EPAC Inhibitors
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2017-10-02 00:00:00 , DOI: 10.1021/acsmedchemlett.7b00358 Yogesh A. Sonawane , Yingmin Zhu 1 , Jered C. Garrison , Edward L. Ezell , Muhammad Zahid , Xiaodong Cheng 1 , Amarnath Natarajan
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2017-10-02 00:00:00 , DOI: 10.1021/acsmedchemlett.7b00358 Yogesh A. Sonawane , Yingmin Zhu 1 , Jered C. Garrison , Edward L. Ezell , Muhammad Zahid , Xiaodong Cheng 1 , Amarnath Natarajan
Affiliation
|
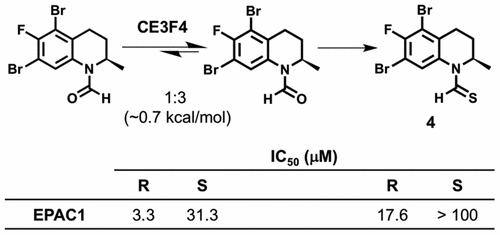
EPAC proteins are therapeutic targets for the potential treatment of cardiac hypertrophy and cancer metastasis. Several laboratories use a tetrahydroquinoline analog, CE3F4, to dissect the role of EPAC1 in various disease states. Here, we report SAR studies with tetrahydroquinoline analogs that explore various functional groups. The most potent EPAC inhibitor 12a exists as a mixture of inseparable E (major) and Z (minor) rotamers. The rotation about the N-formyl group indeed impacts the activity against EPAC.
中文翻译:

四氢喹啉类似物作为EPAA抑制剂的结构-活性关系研究
EPAC蛋白是潜在治疗心脏肥大和癌症转移的治疗靶标。一些实验室使用四氢喹啉类似物CE3F4来剖析EPAC1在各种疾病状态中的作用。在这里,我们报道了使用四氢喹啉类似物探索各种功能基团的SAR研究。最有效的EPAC抑制剂12a以不可分离的E(主要)和Z(次要)旋转异构体的混合物形式存在。围绕N-甲酰基的旋转确实影响了针对EPAC的活性。
更新日期:2017-10-02
中文翻译:

四氢喹啉类似物作为EPAA抑制剂的结构-活性关系研究
EPAC蛋白是潜在治疗心脏肥大和癌症转移的治疗靶标。一些实验室使用四氢喹啉类似物CE3F4来剖析EPAC1在各种疾病状态中的作用。在这里,我们报道了使用四氢喹啉类似物探索各种功能基团的SAR研究。最有效的EPAC抑制剂12a以不可分离的E(主要)和Z(次要)旋转异构体的混合物形式存在。围绕N-甲酰基的旋转确实影响了针对EPAC的活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号