当前位置:
X-MOL 学术
›
Eur. Polym. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Understanding cyclic by-products and ether linkage formation pathways in the transesterification synthesis of aliphatic polycarbonates
European Polymer Journal ( IF 5.8 ) Pub Date : 2017-12-01 , DOI: 10.1016/j.eurpolymj.2017.10.019 Qiang He , Qing Zhang , Shurui Liao , Changsheng Zhao , Xingyi Xie
European Polymer Journal ( IF 5.8 ) Pub Date : 2017-12-01 , DOI: 10.1016/j.eurpolymj.2017.10.019 Qiang He , Qing Zhang , Shurui Liao , Changsheng Zhao , Xingyi Xie
|
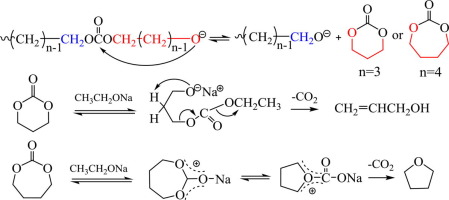
Abstract An overall picture of the reaction pathways towards the side products and final polymers during the transesterification synthesis of biodegradable aliphatic carbonates (APCs) were delineated for the first time. Catalysed by sodium ethoxide, the normal reaction between α,ω-diols (carbon number n = 2–6) and diethyl carbonate generates APC oligomers with propagating terminal alkoxide anions. These anions can backbite at the nearest carbonate carbons or the nearest carbonate α-CH 2 carbons in the same APC chains, eliminating corresponding cyclic carbonates or cyclic ethers. Also, each alkoxide anion can randomly attack a carbonate α-CH 2 carbon in another APC chain, generating ether linkages in the final polymers whose percentages are about 100%, 1.35%, 0.08% and 0% for n = 2, 3, 4 and 5, respectively. The content of cyclic carbonates shows a similar decreasing trend; ethylene carbonate (n = 2) even predominates over the final polymer while trimethylene carbonate (n = 3) and 1,4-butylene carbonate (n = 4) are only minor by-products. Moreover, the latter two cyclic carbonates are not stable enough and can further decarboxylate, forming relatively high amount of allyl alcohol and tetrahydrofuran, respectively. However, trimethylene oxide (n = 3) and tetrahydropyran (n = 5) are minor cyclic ethers. The thorough understanding of the chemical process is essential to improve the APC synthesis.
中文翻译:

了解脂肪族聚碳酸酯酯交换合成中的环状副产物和醚键形成途径
摘要 首次描绘了可生物降解脂肪族碳酸酯 (APC) 酯交换合成过程中副产物和最终聚合物的反应途径的总体情况。在乙醇钠的催化下,α,ω-二醇(碳数 n = 2-6)和碳酸二乙酯之间的正常反应会生成具有传播末端醇盐阴离子的 APC 低聚物。这些阴离子可以在相同 APC 链中最近的碳酸酯碳或最近的碳酸酯 α-CH 2 碳上反咬合,从而消除相应的环状碳酸酯或环状醚。此外,每个醇盐阴离子可以随机攻击另一个 APC 链中的碳酸酯 α-CH 2 碳,在最终聚合物中产生醚键,其百分比约为 100%、1.35%、0.08% 和 0%,n = 2、3、4和 5,分别。环状碳酸酯的含量也呈现类似的下降趋势;碳酸亚乙酯 (n = 2) 甚至在最终聚合物中占主导地位,而碳酸亚丙酯 (n = 3) 和 1,4-碳酸亚丁酯 (n = 4) 只是次要的副产物。此外,后两种环状碳酸酯不够稳定,可以进一步脱羧,分别形成相对大量的烯丙醇和四氢呋喃。然而,环氧丙烷 (n = 3) 和四氢吡喃 (n = 5) 是次要的环醚。对化学过程的透彻理解对于改进 APC 合成至关重要。后两种环状碳酸酯不够稳定,可以进一步脱羧,分别形成相对大量的烯丙醇和四氢呋喃。然而,环氧丙烷 (n = 3) 和四氢吡喃 (n = 5) 是次要的环醚。对化学过程的透彻理解对于改进 APC 合成至关重要。后两种环状碳酸酯不够稳定,可以进一步脱羧,分别形成相对大量的烯丙醇和四氢呋喃。然而,环氧丙烷 (n = 3) 和四氢吡喃 (n = 5) 是次要的环醚。对化学过程的透彻理解对于改进 APC 合成至关重要。
更新日期:2017-12-01
中文翻译:

了解脂肪族聚碳酸酯酯交换合成中的环状副产物和醚键形成途径
摘要 首次描绘了可生物降解脂肪族碳酸酯 (APC) 酯交换合成过程中副产物和最终聚合物的反应途径的总体情况。在乙醇钠的催化下,α,ω-二醇(碳数 n = 2-6)和碳酸二乙酯之间的正常反应会生成具有传播末端醇盐阴离子的 APC 低聚物。这些阴离子可以在相同 APC 链中最近的碳酸酯碳或最近的碳酸酯 α-CH 2 碳上反咬合,从而消除相应的环状碳酸酯或环状醚。此外,每个醇盐阴离子可以随机攻击另一个 APC 链中的碳酸酯 α-CH 2 碳,在最终聚合物中产生醚键,其百分比约为 100%、1.35%、0.08% 和 0%,n = 2、3、4和 5,分别。环状碳酸酯的含量也呈现类似的下降趋势;碳酸亚乙酯 (n = 2) 甚至在最终聚合物中占主导地位,而碳酸亚丙酯 (n = 3) 和 1,4-碳酸亚丁酯 (n = 4) 只是次要的副产物。此外,后两种环状碳酸酯不够稳定,可以进一步脱羧,分别形成相对大量的烯丙醇和四氢呋喃。然而,环氧丙烷 (n = 3) 和四氢吡喃 (n = 5) 是次要的环醚。对化学过程的透彻理解对于改进 APC 合成至关重要。后两种环状碳酸酯不够稳定,可以进一步脱羧,分别形成相对大量的烯丙醇和四氢呋喃。然而,环氧丙烷 (n = 3) 和四氢吡喃 (n = 5) 是次要的环醚。对化学过程的透彻理解对于改进 APC 合成至关重要。后两种环状碳酸酯不够稳定,可以进一步脱羧,分别形成相对大量的烯丙醇和四氢呋喃。然而,环氧丙烷 (n = 3) 和四氢吡喃 (n = 5) 是次要的环醚。对化学过程的透彻理解对于改进 APC 合成至关重要。











































 京公网安备 11010802027423号
京公网安备 11010802027423号