当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Tetra(3‐thienyl)biphenoquinone and its Charge Transfer Complex with Perylene
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2017-11-16 , DOI: 10.1002/ajoc.201700560 Ryotaro Fujii 1 , Md. Awlad Hossain 1 , Hisato Akimoto 1 , Kazunori Hirabayashi 1 , Toshio Shimizu 1 , Kazuhiko Akiyama 1 , Kenta Goto 2 , Hiroyuki Nishikawa 3 , Ken-ichi Yamashita 4 , Ken-ichi Sugiura 1
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2017-11-16 , DOI: 10.1002/ajoc.201700560 Ryotaro Fujii 1 , Md. Awlad Hossain 1 , Hisato Akimoto 1 , Kazunori Hirabayashi 1 , Toshio Shimizu 1 , Kazuhiko Akiyama 1 , Kenta Goto 2 , Hiroyuki Nishikawa 3 , Ken-ichi Yamashita 4 , Ken-ichi Sugiura 1
Affiliation
|
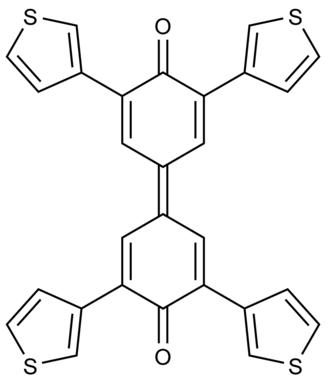
Tetra(thienyl)biphenoquinones were designed and synthesized. An oxidative coupling of 2,6‐bis(3‐thienyl)phenol with PbO2 in acetic acid afforded the corresponding biphenoquinone along with 2,6‐bis(3‐thienyl)‐p‐benzoquinone. This biphenoquinone showed properties characteristic of a π‐expanded quinone, that is, positively shifted reduction potential and light absorption in the visible region. In contrast to kinetically protected biphenoquinones with bulky substituents at the ortho‐positions of carbonyls, the title compounds take a planar structure because of the small steric repulsion of the five‐membered thiophene rings. Its charge transfer complex with perylene is characterized by a well‐overlapped mixed stack.
中文翻译:

四(3-噻吩基)联苯醌的合成及其与Per的电荷转移配合物
设计并合成了四(噻吩基)联苯醌。在乙酸中2,6-双(3-噻吩基)苯酚与PbO 2的氧化偶合提供了相应的联苯醌和2,6-双(3-噻吩基)-对-苯醌。该联苯醌显示了π扩展醌的特性,即在可见光区域正还原电位正移和光吸收。与在羰基邻位具有大取代基的动力学保护联苯醌相比,由于五元噻吩环的空间排斥力小,因此标题化合物呈平面结构。其与per的电荷转移复合物的特征是重叠良好的混合烟囱。
更新日期:2017-11-16
中文翻译:

四(3-噻吩基)联苯醌的合成及其与Per的电荷转移配合物
设计并合成了四(噻吩基)联苯醌。在乙酸中2,6-双(3-噻吩基)苯酚与PbO 2的氧化偶合提供了相应的联苯醌和2,6-双(3-噻吩基)-对-苯醌。该联苯醌显示了π扩展醌的特性,即在可见光区域正还原电位正移和光吸收。与在羰基邻位具有大取代基的动力学保护联苯醌相比,由于五元噻吩环的空间排斥力小,因此标题化合物呈平面结构。其与per的电荷转移复合物的特征是重叠良好的混合烟囱。











































 京公网安备 11010802027423号
京公网安备 11010802027423号