当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Identifying Dysregulated Epigenetic Enzyme Activity in Castrate-Resistant Prostate Cancer Development
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2017-10-11 00:00:00 , DOI: 10.1021/acschembio.6b01035 Jin-Hee Lee 1, 2 , Bing Yang 3 , Anastasia J. Lindahl 1, 2 , Nathan Damaschke 3 , Melissa D. Boersma 2, 4 , Wei Huang 5 , Eva Corey 6 , David F. Jarrard 3, 7, 8 , John M. Denu 1, 2, 7
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2017-10-11 00:00:00 , DOI: 10.1021/acschembio.6b01035 Jin-Hee Lee 1, 2 , Bing Yang 3 , Anastasia J. Lindahl 1, 2 , Nathan Damaschke 3 , Melissa D. Boersma 2, 4 , Wei Huang 5 , Eva Corey 6 , David F. Jarrard 3, 7, 8 , John M. Denu 1, 2, 7
Affiliation
|
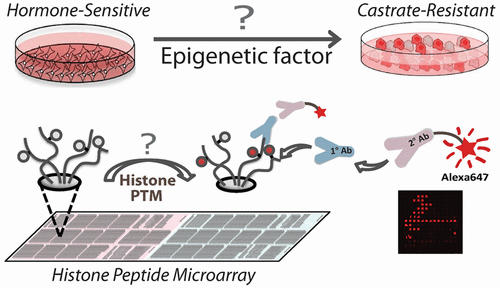
There is a tremendous need for novel strategies aimed at directly assessing activities of histone modifiers to probe epigenetic determinants associated with disease progression. Here, we developed a high-throughput peptide microarray assay to identify altered histone lysine (de)acetylation activity in prostate cancer (PCa). This microarray-based activity assay revealed up-regulated histone acetyltransferase (HAT) activity against specific histone H3 sites in a castrate-resistant (CR) PCa cell line compared to its hormone-sensitive (HS) isogenic counterpart. NAD+-dependent deacetylation assays revealed down-regulated sirtuin activity in validated CR lines. Levels of acetyltransferases GCN5, PCAF, CBP, and p300 were unchanged between matched HS and CR cell lines. However, autoacetylation of p300 at K1499, a modification known to enhance HAT activity and a target of deacetylation by SIRT2, was highly elevated in CR cells, while SIRT2 protein level was reduced in CR cells. Interrogation of HS and matched CR xenograft lines reveals that H3K18 hyperacetylation, increased p300 activity, and decreased SIRT2 expression are associated with progression to CR in 8/12 (66%). Tissue microarray analysis revealed that hyperacetylation of H3K18 is a feature of CRPC. Inhibition of p300 results in lower H3K18ac levels and increased expression of androgen receptors. Thus, a novel histone array identifies altered enzyme activities during the progression to CRPC and may be utilized in a personalized medicine approach. Reduced SIRT2 expression and increased p300 activity lead to a concerted mechanism of hyperacetylation at specific histone lysine sites (H3K9, H3K14, and H3K18) in CRPC.
中文翻译:

在去势抵抗的前列腺癌发展中识别失调的表观遗传酶活性。
迫切需要旨在直接评估组蛋白修饰剂的活性以探测与疾病进展相关的表观遗传决定因素的新颖策略。在这里,我们开发了一种高通量肽微阵列测定法,以鉴定前列腺癌(PCa)中组蛋白赖氨酸(去)乙酰化活性的改变。这项基于微阵列的活性分析表明,与其去皮激素抵抗(HS)等基因对应物相比,去势抵抗(CR)PCa细胞系中针对特定组蛋白H3位点的组蛋白乙酰转移酶(HAT)活性上调。NAD +依赖的脱乙酰化试验显示在经过验证的CR品系中Sirtuin活性下调。在匹配的HS和CR细胞系之间,乙酰转移酶GCN5,PCAF,CBP和p300的水平没有变化。但是,CR细胞中p300在K1499的自乙酰化(一种已知增强HAT活性和SIRT2脱乙酰作用的靶点的修饰)在CR细胞中高度升高,而SIRT2蛋白水平在CR细胞中降低。对HS和匹配的CR异种移植物系的询问表明,H3K18过度乙酰化,p300活性增加和SIRT2表达降低与8/12的CR进展相关(66%)。组织微阵列分析表明,H3K18的超乙酰化是CRPC的特征。抑制p300会降低H3K18ac的水平,并增加雄激素受体的表达。因此,一种新颖的组蛋白阵列可确定在发展为CRPC的过程中酶活性的变化,并可用于个性化医学方法。SIRPC2表达减少和p300活性增加导致CRPC中特定组蛋白赖氨酸位点(H3K9,H3K14和H3K18)发生超乙酰化的协同机制。
更新日期:2017-10-11
中文翻译:

在去势抵抗的前列腺癌发展中识别失调的表观遗传酶活性。
迫切需要旨在直接评估组蛋白修饰剂的活性以探测与疾病进展相关的表观遗传决定因素的新颖策略。在这里,我们开发了一种高通量肽微阵列测定法,以鉴定前列腺癌(PCa)中组蛋白赖氨酸(去)乙酰化活性的改变。这项基于微阵列的活性分析表明,与其去皮激素抵抗(HS)等基因对应物相比,去势抵抗(CR)PCa细胞系中针对特定组蛋白H3位点的组蛋白乙酰转移酶(HAT)活性上调。NAD +依赖的脱乙酰化试验显示在经过验证的CR品系中Sirtuin活性下调。在匹配的HS和CR细胞系之间,乙酰转移酶GCN5,PCAF,CBP和p300的水平没有变化。但是,CR细胞中p300在K1499的自乙酰化(一种已知增强HAT活性和SIRT2脱乙酰作用的靶点的修饰)在CR细胞中高度升高,而SIRT2蛋白水平在CR细胞中降低。对HS和匹配的CR异种移植物系的询问表明,H3K18过度乙酰化,p300活性增加和SIRT2表达降低与8/12的CR进展相关(66%)。组织微阵列分析表明,H3K18的超乙酰化是CRPC的特征。抑制p300会降低H3K18ac的水平,并增加雄激素受体的表达。因此,一种新颖的组蛋白阵列可确定在发展为CRPC的过程中酶活性的变化,并可用于个性化医学方法。SIRPC2表达减少和p300活性增加导致CRPC中特定组蛋白赖氨酸位点(H3K9,H3K14和H3K18)发生超乙酰化的协同机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号