当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of 2,3,4,5-Tetrahydrobenzo[1,4]thiazepines via N-Acyliminium Cyclization
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2017-10-09 00:00:00 , DOI: 10.1021/acs.oprd.7b00260 Shixian Deng 1, 2 , Zhenzhuang Cheng 1 , Charles Ingalls 1 , Ferenc Kontes 1 , Jiaming Yan 1 , Sandro Belvedere 1
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2017-10-09 00:00:00 , DOI: 10.1021/acs.oprd.7b00260 Shixian Deng 1, 2 , Zhenzhuang Cheng 1 , Charles Ingalls 1 , Ferenc Kontes 1 , Jiaming Yan 1 , Sandro Belvedere 1
Affiliation
|
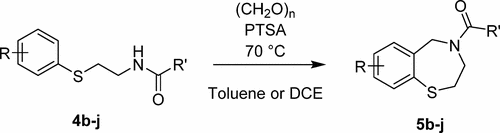
We report an efficient and scalable synthesis of 7-methoxy-2,3,4,5-tetrahydrobenzo[1,4]thiazepine, the core structure of biologically active molecules like JTV-519 and S107. This synthetic route, starting with 4-methoxythiophenol and proceeding via acyliminum cyclization, gives the target product in four steps and 68% overall yield and is a substantial improvement over previously published processes. Nine additional examples of tetrahydrobenzo[1,4]thiazepine synthesis via acyliminium ring closure are also presented.
中文翻译:

N-酰基亚胺基环化反应合成2,3,4,5-四氢苯并[1,4]噻嗪类
我们报告了7-甲氧基-2,3,4,5-四氢苯并[1,4]硫氮平的有效和可扩展的合成,该生物活性分子如JTV-519和S107的核心结构。该合成路线从4-甲氧基硫酚开始,并通过亚胺基环化进行,分四个步骤提供目标产物,总收率达68%,与以前公布的工艺相比有实质性的改进。还提供了通过酰基亚胺环闭环合成四氢苯并[1,4]噻氮平的另外九个实例。
更新日期:2017-10-09
中文翻译:

N-酰基亚胺基环化反应合成2,3,4,5-四氢苯并[1,4]噻嗪类
我们报告了7-甲氧基-2,3,4,5-四氢苯并[1,4]硫氮平的有效和可扩展的合成,该生物活性分子如JTV-519和S107的核心结构。该合成路线从4-甲氧基硫酚开始,并通过亚胺基环化进行,分四个步骤提供目标产物,总收率达68%,与以前公布的工艺相比有实质性的改进。还提供了通过酰基亚胺环闭环合成四氢苯并[1,4]噻氮平的另外九个实例。



























 京公网安备 11010802027423号
京公网安备 11010802027423号