当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Regioselective C5 alkenylation of 2-acylpyrroles via Pd(ii)-catalyzed C–H bond activation†
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2017-10-09 00:00:00 , DOI: 10.1039/c7qo00732a Jian-Hua Duan 1, 2, 3, 4 , Rui-Jie Mi 1, 2, 3, 4 , Jing Sun 1, 2, 3, 4 , Ming-Dong Zhou 1, 2, 3, 4
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2017-10-09 00:00:00 , DOI: 10.1039/c7qo00732a Jian-Hua Duan 1, 2, 3, 4 , Rui-Jie Mi 1, 2, 3, 4 , Jing Sun 1, 2, 3, 4 , Ming-Dong Zhou 1, 2, 3, 4
Affiliation
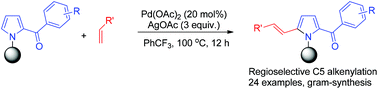
|
A Pd(II)-catalyzed regioselective C5 alkenylation of 2-acylpyrroles has been developed employing 3-nitrile benzoyl group (N-(3-NCC6H4CO-)) as an efficient N-protecting group. The protocol provided a simple and efficient method to synthesize C5-alkenylated 2-acylpyrrole derivatives. The employed N-protecting group was readily removable in the presence of HCl/EtOH under mild conditions.
中文翻译:

通过Pd(ii)催化的C–H键活化, 对2-酰基吡咯的区域选择性C5烯基化作用†
已经开发了使用3-腈苯甲酰基(N-(3-NCC 6 H 4 CO-))作为有效的N-保护基的Pd(II)催化的2-酰基吡咯的区域选择性C5烯基化。该协议提供了一种简单有效的方法来合成C5-烯基化的2-酰基吡咯衍生物。所用的N-保护基在温和条件下在HCl / EtOH存在下易于除去。
更新日期:2017-10-09
中文翻译:

通过Pd(ii)催化的C–H键活化, 对2-酰基吡咯的区域选择性C5烯基化作用†
已经开发了使用3-腈苯甲酰基(N-(3-NCC 6 H 4 CO-))作为有效的N-保护基的Pd(II)催化的2-酰基吡咯的区域选择性C5烯基化。该协议提供了一种简单有效的方法来合成C5-烯基化的2-酰基吡咯衍生物。所用的N-保护基在温和条件下在HCl / EtOH存在下易于除去。











































 京公网安备 11010802027423号
京公网安备 11010802027423号