当前位置:
X-MOL 学术
›
J. Fluorine Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of trifluoromethylated pyrrolidines via decarboxylative [3+2] cycloaddition of non-stabilized N-unsubstituted azomethine ylides
Journal of Fluorine Chemistry ( IF 1.7 ) Pub Date : 2017-10-07 , DOI: 10.1016/j.jfluchem.2017.10.003 Xiaofeng Zhang , Miao Liu , Wensheng Zhang , Marc Legris , Wei Zhang
中文翻译:

通过非稳定的N-未取代的偶氮甲亚胺的脱羧[3 + 2]环加成反应合成三氟甲基化的吡咯烷
更新日期:2017-10-07
Journal of Fluorine Chemistry ( IF 1.7 ) Pub Date : 2017-10-07 , DOI: 10.1016/j.jfluchem.2017.10.003 Xiaofeng Zhang , Miao Liu , Wensheng Zhang , Marc Legris , Wei Zhang
|
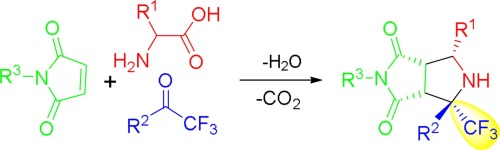
A new method for synthesis of trifluoromethylated and pyrrolidinedione-fused pyrrolidines is developed through a three-component and decarboxylative [3 + 2] cycloaddition of non-stabilized N-unsubstituted azomethine ylides with commercially available trifluoroacetophenones. This efficient and economic synthesis only produces stoichiometric amount of CO2 and H2O as byproducts.
中文翻译:

通过非稳定的N-未取代的偶氮甲亚胺的脱羧[3 + 2]环加成反应合成三氟甲基化的吡咯烷
通过将未稳定的N-未取代的偶氮甲亚胺与市场上可得的三氟苯乙酮进行三组分和脱羧[3 + 2]环加成反应,开发了一种新的合成三氟甲基化和吡咯烷二酮稠合的吡咯烷的方法。这种高效和经济的合成仅产生CO的化学计量量的2和H 2 O作为副产物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号