Journal of Fluorine Chemistry ( IF 1.7 ) Pub Date : 2017-10-04 , DOI: 10.1016/j.jfluchem.2017.09.011 Karl O. Christe , Ralf Haiges , Martin Rahm , David A. Dixon , Monica Vasiliu
|
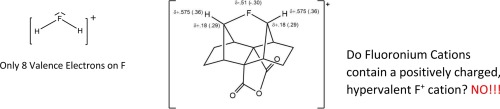
In a recent publication (Science, 2013, 340, 57), indirect experimental evidence was presented for the transient generation in solution of a fluorine atom symmetrically bonded to two carbon atoms. The bridging atom was described as a formally positively charged fluorine engaged in hypervalent bonding, and the molecule was referred to as a fluoronium ion. This interpretation was emphasized in chemistryworld “as the first evidence for hypervalent fluorine cations, or fluoronium ions” (chemistryworld, 4 April, 2013), in Chemical & Engineering News as “fluorine’s positive side revealed” (C&E News, 2013, 91, (14) 36), and in a Science perspective as “revealing the positive side of fluorine” (Science, 2013, 340, 41). A critical examination of these interpretations shows that this symmetrically bonded fluorine atom does not carry a positive charge and is not hypervalent or hypercoordinate. Furthermore, it is shown that, in contrast to the “fluoronium ions” which always contain partially negatively charged fluorine atoms, the iodonium cations are true halonium ions according to the IUPAC definition which requires a positive charge on the halogen atom. The calculated partial charges on the halogen atoms in chloronium and bromonium cations are method dependent and can vary from compound to compound, and therefore are more ambiguous.
中文翻译:

对氟离子和高价氟阳离子的误解
在最近的出版物(《科学》, 2013年,第 340页,第57页)中,提供了间接实验证据来证明在溶液中瞬时生成对称结合到两个碳原子上的氟原子。桥接原子被描述为参与高价键的形式带正电荷的氟,并且该分子被称为氟离子。这种解释强调在chemistryworld “作为第一个证据高价氟离子,或二氢氟阳离子离子”(chemistryworld 4月4日, 2013),在化学化工新闻为“氟的积极的一面显露”(C&E新闻,2013,91,(14)36),并在科学界的观点是“揭示氟的积极方面”(《科学》,2013年,340页,41)。对这些解释的严格检查表明,该对称键合的氟原子不带正电荷,也不是高价或超配位的。此外,显示出,与总是包含部分带负电荷的氟原子的“氟离子”相反,根据IUPAC的定义,碘鎓阳离子是真正的ha离子,其需要在卤原子上带正电荷。所计算的氯鎓和溴阳离子中卤素原子上的部分电荷与方法有关,并且可能因化合物而异,因此更加不明确。










































 京公网安备 11010802027423号
京公网安备 11010802027423号