当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, Synthesis, and Antibacterial Evaluation of Oxazolidinones with Fused Heterocyclic C-Ring Substructure
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2017-10-05 00:00:00 , DOI: 10.1021/acsmedchemlett.7b00263 Mahesh S. Deshmukh 1, 2 , Nidhi Jain 2
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2017-10-05 00:00:00 , DOI: 10.1021/acsmedchemlett.7b00263 Mahesh S. Deshmukh 1, 2 , Nidhi Jain 2
Affiliation
|
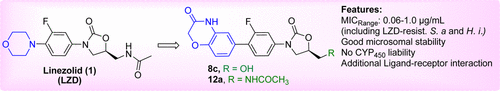
A series of novel oxazolidinone antibacterials with diverse fused heteroaryl C-rings bearing hydrogen bond donor and hydrogen bond acceptor functionalities were designed and synthesized. The compound with benzoxazinone C-ring substructure (8c) exhibited superior activity compared to linezolid against a panel of Gram-positive and Gram-negative bacteria. Structural modifications at C5-side chain of 8c resulted in identification of several potent compounds (12a, 12b, 12g, and 12h). Selected compounds 8c and 12a showed very good microsomal stability and no CYP450 liability, thus clearing preliminary safety hurdles. A docking model of 12a binding to 23S rRNA suggested that the increased potency of 12a is due to additional ligand–receptor interaction.
中文翻译:

稠合杂环C环亚结构的恶唑烷酮的设计,合成及抗菌性能评价
设计并合成了一系列新颖的恶唑烷酮类抗菌剂,它们具有带有氢键供体和氢键受体功能的各种稠合杂芳基C环。与利奈唑胺相比,具有苯并恶嗪酮C环亚结构的化合物(8c)对一组革兰氏阳性和革兰氏阴性细菌表现出更高的活性。8c的C5侧链的结构修饰导致鉴定了几种有效的化合物(12a,12b,12g和12h)。选定的化合物8c和12a显示出非常好的微粒体稳定性,并且没有CYP 450责任,从而消除了初步的安全障碍。的对接模型12A结合23S rRNA的建议的增强的效力12A是由于附加的配体-受体相互作用。
更新日期:2017-10-05
中文翻译:

稠合杂环C环亚结构的恶唑烷酮的设计,合成及抗菌性能评价
设计并合成了一系列新颖的恶唑烷酮类抗菌剂,它们具有带有氢键供体和氢键受体功能的各种稠合杂芳基C环。与利奈唑胺相比,具有苯并恶嗪酮C环亚结构的化合物(8c)对一组革兰氏阳性和革兰氏阴性细菌表现出更高的活性。8c的C5侧链的结构修饰导致鉴定了几种有效的化合物(12a,12b,12g和12h)。选定的化合物8c和12a显示出非常好的微粒体稳定性,并且没有CYP 450责任,从而消除了初步的安全障碍。的对接模型12A结合23S rRNA的建议的增强的效力12A是由于附加的配体-受体相互作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号