当前位置:
X-MOL 学术
›
J. Fluorine Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
α-Trifluoromethylseleno-substituted ketones synthesis via copper-mediated trifluoromethylselenolation of α-bromoketones
Journal of Fluorine Chemistry ( IF 1.7 ) Pub Date : 2017-10-03 , DOI: 10.1016/j.jfluchem.2017.10.001 Yue Yang , Xia Lin , Zhiwei Zheng , Ganglu Lin , Yunxiao Zhang , Yi You , Zhiqiang Weng
中文翻译:

α-Trifluoromethylseleno取代酮合成经由α-溴酮的铜介导的trifluoromethylselenolation
更新日期:2017-10-03
Journal of Fluorine Chemistry ( IF 1.7 ) Pub Date : 2017-10-03 , DOI: 10.1016/j.jfluchem.2017.10.001 Yue Yang , Xia Lin , Zhiwei Zheng , Ganglu Lin , Yunxiao Zhang , Yi You , Zhiqiang Weng
|
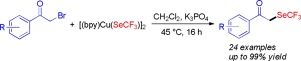
Herein we disclose the report on the synthesis of α-trifluoromethylseleno-substituted ketone derivatives via copper-mediated trifluoromethylselenolation of α-bromoketones with [(bpy)Cu(SeCF3)]2 1. A broad range of substituted 2-bromoacetophenone substrates were found to react smoothly with 1 in the presence of 3 equiv of K3PO4 to afford the corresponding α-trifluoromethylseleno-substituted ketones in good yields. A wide range of functional groups were well tolerated and the reaction conditions are amenable to scale up.
中文翻译:

α-Trifluoromethylseleno取代酮合成经由α-溴酮的铜介导的trifluoromethylselenolation
在这里,我们公开了通过[(bpy)Cu(SeCF 3)] 2 1通过铜介导的α-溴代酮的三氟甲基硒化反应合成α-三氟甲基硒代酮衍生物的报告。发现了广泛的取代2-溴苯乙酮底物在3当量的K 3 PO 4的存在下,与1平滑反应,以高收率得到相应的α-三氟甲基硒代取代的酮。各种各样的官能团被很好地耐受,并且反应条件适合扩大规模。











































 京公网安备 11010802027423号
京公网安备 11010802027423号