当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Sequential asymmetric hydrogenation and photoredox chemistry with a single catalyst†
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2017-10-04 00:00:00 , DOI: 10.1039/c7qo00784a Xiao Zhang 1, 2, 3, 4 , Jie Qin 1, 2, 3, 4 , Xiaoqiang Huang 1, 2, 3, 4 , Eric Meggers 1, 2, 3, 4
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2017-10-04 00:00:00 , DOI: 10.1039/c7qo00784a Xiao Zhang 1, 2, 3, 4 , Jie Qin 1, 2, 3, 4 , Xiaoqiang Huang 1, 2, 3, 4 , Eric Meggers 1, 2, 3, 4
Affiliation
|
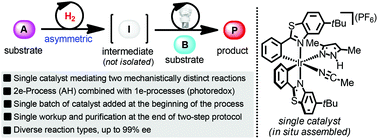
An important objective in organic synthesis is the generation of structural complexity in a straightforward and economical fashion. Herein, we address this challenge by reporting a process in which a single chiral iridium catalyst promotes two mechanistically distinct reaction types in a sequential fashion, namely asymmetric hydrogenation (two-electron mechanism) and photoredox chemistry (one-electron mechanism). A variety of chiral alcohols are generated with enantioselectivities of 91–99% ee using a single batch of catalyst added at the beginning of the reaction sequence without any requirement for isolating the reaction intermediates.
中文翻译:

单一催化剂的顺序不对称加氢和光氧化还原化学反应†
有机合成的一个重要目标是以直接和经济的方式产生结构复杂性。在本文中,我们通过报告一种过程来应对这一挑战,其中单个手性铱催化剂以不对称氢化(双电子机理)和光氧化还原化学(单电子机理)的顺序方式促进两种机理不同的反应类型。使用一批在反应顺序开始时加入的催化剂,可以产生对映选择性为91–99%ee的多种手性醇,而无需分离任何反应中间体。
更新日期:2017-10-04
中文翻译:

单一催化剂的顺序不对称加氢和光氧化还原化学反应†
有机合成的一个重要目标是以直接和经济的方式产生结构复杂性。在本文中,我们通过报告一种过程来应对这一挑战,其中单个手性铱催化剂以不对称氢化(双电子机理)和光氧化还原化学(单电子机理)的顺序方式促进两种机理不同的反应类型。使用一批在反应顺序开始时加入的催化剂,可以产生对映选择性为91–99%ee的多种手性醇,而无需分离任何反应中间体。











































 京公网安备 11010802027423号
京公网安备 11010802027423号