当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
One-flask synthesis of dibenzotetraaza[14]annulene cyclic congeners bearing buta-1,3-diyne bridges†
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2017-09-27 00:00:00 , DOI: 10.1039/c7qo00821j K. M. Zwoliński 1, 2, 3, 4 , L. Sieroń 4, 5, 6, 7 , J. Eilmes 1, 2, 3, 4
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2017-09-27 00:00:00 , DOI: 10.1039/c7qo00821j K. M. Zwoliński 1, 2, 3, 4 , L. Sieroń 4, 5, 6, 7 , J. Eilmes 1, 2, 3, 4
Affiliation
|
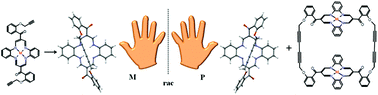
A macrocyclic precursor featuring highly reactive propargylic moieties was prepared via Williamson etherification in good yields of 54–65%. Organometallic methodology paired with protective metal insertion served as the foundation for better DBTAA exploitation through copper(I)-mediated coupling. Glaser–Hay and Glaser–Eglinton coupling conditions were applied to the direct synthesis of a cyclic strapped ligand and its corresponding dimer in 44% and 30% yields respectively. Mechanistic insight into cyclic dimer formation is discussed with emphasis on the kinetic effective molarities which reveal that the working concentration regime profoundly affects the final product's distribution. Both macrocyclic zinc(II) precursor and the corresponding inherently chiral strapped ligand are structurally characterized by single-crystal X-ray diffraction. Strapped enantiomers communicate in a solid state through the inherently chiral π-surfaces and display preferential self-recognition, leading to both homochiral MM and PP stereoisomers with pronounced helicity.
中文翻译:

单烧瓶合成带有buta-1,3-diyne桥的二苯并四氮杂[14]年环环同类物†
通过Williamson醚化制备了具有高反应性炔丙基部分的大环前体,收率为54–65%。有机金属方法与保护性金属插入相结合,是通过铜(I)介导的偶联更好地利用DBTAA的基础。将Glaser-Hay和Glaser-Eglinton偶联条件分别用于环状绑带配体及其相应二聚体的直接合成,产率分别为44%和30%。讨论了对环状二聚体形成的机理认识,并着重于动力学有效摩尔浓度,揭示了工作浓度机制对最终产物的分布产生了深远的影响。均大环锌(Ⅱ前体和相应的固有手性束缚的配体通过单晶X射线衍射在结构上表征。束缚的对映异构体通过固有的手性π表面以固态进行通讯并显示优先的自我识别,从而导致具有明显螺旋度的同手性MM和PP立体异构体。
更新日期:2017-09-27
中文翻译:

单烧瓶合成带有buta-1,3-diyne桥的二苯并四氮杂[14]年环环同类物†
通过Williamson醚化制备了具有高反应性炔丙基部分的大环前体,收率为54–65%。有机金属方法与保护性金属插入相结合,是通过铜(I)介导的偶联更好地利用DBTAA的基础。将Glaser-Hay和Glaser-Eglinton偶联条件分别用于环状绑带配体及其相应二聚体的直接合成,产率分别为44%和30%。讨论了对环状二聚体形成的机理认识,并着重于动力学有效摩尔浓度,揭示了工作浓度机制对最终产物的分布产生了深远的影响。均大环锌(Ⅱ前体和相应的固有手性束缚的配体通过单晶X射线衍射在结构上表征。束缚的对映异构体通过固有的手性π表面以固态进行通讯并显示优先的自我识别,从而导致具有明显螺旋度的同手性MM和PP立体异构体。











































 京公网安备 11010802027423号
京公网安备 11010802027423号