当前位置:
X-MOL 学术
›
ACS Infect. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure–Activity and −Toxicity Relationships of the Antimicrobial Peptide Tachyplesin-1
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2017-10-03 00:00:00 , DOI: 10.1021/acsinfecdis.7b00123 Ingrid A. Edwards 1 , Alysha G. Elliott 1 , Angela M. Kavanagh 1 , Mark A. T. Blaskovich 1 , Matthew A. Cooper 1
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2017-10-03 00:00:00 , DOI: 10.1021/acsinfecdis.7b00123 Ingrid A. Edwards 1 , Alysha G. Elliott 1 , Angela M. Kavanagh 1 , Mark A. T. Blaskovich 1 , Matthew A. Cooper 1
Affiliation
|
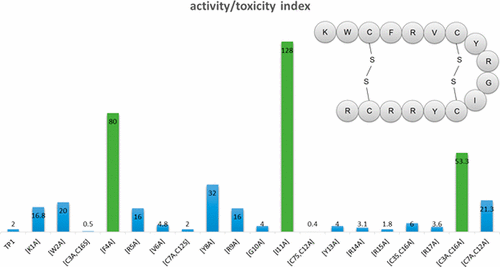
Tachyplesin-1 (TP1; 1) is a cationic β-hairpin antimicrobial peptide with a membranolytic mechanism of action. While it possesses broad-spectrum, potent antimicrobial activity, 1 is highly hemolytic against mammalian erythrocytes, which precludes it from further development. In this study, we report a template-based approach to investigate the structure–function and structure–toxicity relationships of each amino acid of 1. We modulated charge and hydrophobicity by residue modification and truncation of the peptide. Antimicrobial activity was then assessed against six key bacterial pathogens and two fungi, with toxicity profiled against mammalian cells. The internal disulfide bridge Cys7-Cys12 of 1 was shown to play an important role in broad-spectrum antimicrobial activity against all pathogenic strains tested. Novel peptides based on the progenitor were then designed, including 5 (TP1[F4A]), 12 (TP1[I11A]), and 19 (TP1[C3A,C16A]). These had 26- to 64-fold improved activity/toxicity indices and show promise for further development. Structural studies of 5 (TP1[F4A]) and 12 (TP1[I11A]) identified a conserved β-hairpin secondary structure motif correlating with their very high stablility in mouse and human plasma. Membrane binding affinity determined by surface plasmon resonance confirmed their selectivity toward bacterial membranes, but the degree of membrane binding did not correlate with the degree of hemolysis, suggesting that other factors may drive toxicity.
中文翻译:

抗菌肽速激肽-1的结构-活性和-毒性关系。
Tachyplesin-1(TP1; 1)是具有膜分解作用机制的阳离子β-发夹抗菌肽。虽然它具有广谱的有效抗菌活性,但1对哺乳动物的红细胞具有高度的溶血性,因此无法进一步开发。在这项研究中,我们报告了一种基于模板的方法,以研究1的每个氨基酸的结构-功能和结构-毒性关系。我们通过残基修饰和截短肽来调节电荷和疏水性。然后评估针对六种关键细菌病原体和两种真菌的抗菌活性,并针对哺乳动物细胞进行毒性分析。内部二硫键Cys7-Cys12 of 1已证明在针对所有测试的病原菌株的广谱抗菌活性中起着重要作用。然后设计基于祖细胞的新型肽,包括5(TP1 [F4A]),12(TP1 [I11A])和19(TP1 [C3A,C16A])。这些具有26至64倍的改善的活性/毒性指数,并显示出进一步发展的希望。5(TP1 [F4A])和12的结构研究(TP1 [I11A])鉴定了一个保守的β-发夹二级结构基序,与它们在小鼠和人血浆中的极高稳定性相关。通过表面等离振子共振测定的膜结合亲和力证实了它们对细菌膜的选择性,但是膜结合的程度与溶血的程度不相关,表明其他因素也可能导致毒性。
更新日期:2017-10-03
中文翻译:

抗菌肽速激肽-1的结构-活性和-毒性关系。
Tachyplesin-1(TP1; 1)是具有膜分解作用机制的阳离子β-发夹抗菌肽。虽然它具有广谱的有效抗菌活性,但1对哺乳动物的红细胞具有高度的溶血性,因此无法进一步开发。在这项研究中,我们报告了一种基于模板的方法,以研究1的每个氨基酸的结构-功能和结构-毒性关系。我们通过残基修饰和截短肽来调节电荷和疏水性。然后评估针对六种关键细菌病原体和两种真菌的抗菌活性,并针对哺乳动物细胞进行毒性分析。内部二硫键Cys7-Cys12 of 1已证明在针对所有测试的病原菌株的广谱抗菌活性中起着重要作用。然后设计基于祖细胞的新型肽,包括5(TP1 [F4A]),12(TP1 [I11A])和19(TP1 [C3A,C16A])。这些具有26至64倍的改善的活性/毒性指数,并显示出进一步发展的希望。5(TP1 [F4A])和12的结构研究(TP1 [I11A])鉴定了一个保守的β-发夹二级结构基序,与它们在小鼠和人血浆中的极高稳定性相关。通过表面等离振子共振测定的膜结合亲和力证实了它们对细菌膜的选择性,但是膜结合的程度与溶血的程度不相关,表明其他因素也可能导致毒性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号