当前位置:
X-MOL 学术
›
React. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic investigation of a Ru-catalyzed direct asymmetric reductive amination reaction for a batch or continuous process scale-up: an industrial perspective
Reaction Chemistry & Engineering ( IF 3.4 ) Pub Date : 2017-08-08 00:00:00 , DOI: 10.1039/c7re00055c Shujauddin M. Changi 1, 2, 3, 4 , Tohru Yokozawa 5, 6, 7, 8 , Tetsuya Yamamoto 5, 6, 7, 8 , Hikaru Nakajima 5, 6, 7, 8 , Matthew C. Embry 1, 2, 3, 4 , Radhe Vaid 1, 2, 3, 4 , Carla V. Luciani 1, 2, 3, 4 , Sze-Wing Wong 1, 2, 3, 4 , Martin Johnson 1, 2, 3, 4 , Eric D. Moher 1, 2, 3, 4
Reaction Chemistry & Engineering ( IF 3.4 ) Pub Date : 2017-08-08 00:00:00 , DOI: 10.1039/c7re00055c Shujauddin M. Changi 1, 2, 3, 4 , Tohru Yokozawa 5, 6, 7, 8 , Tetsuya Yamamoto 5, 6, 7, 8 , Hikaru Nakajima 5, 6, 7, 8 , Matthew C. Embry 1, 2, 3, 4 , Radhe Vaid 1, 2, 3, 4 , Carla V. Luciani 1, 2, 3, 4 , Sze-Wing Wong 1, 2, 3, 4 , Martin Johnson 1, 2, 3, 4 , Eric D. Moher 1, 2, 3, 4
Affiliation
|
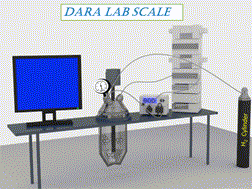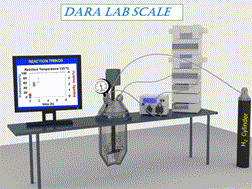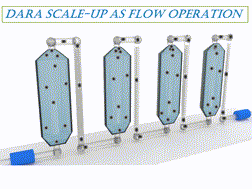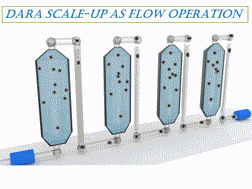
A comprehensive assessment of a Ru-catalyzed direct asymmetric reductive amination (DARA) reaction for producing an intermediate for an active pharmaceutical ingredient (API) was carried out. Experiments were conducted to investigate the impact of process parameters (such as reaction temperature, time, concentration, pressure, Ru-catalyst concentration, acid catalyst, and reagent stoichiometry) on chemo- and stereo-selectivity, and yield. An analysis of experimental data led to the development of a mechanistic mathematical model that was mathematically consistent with data from laboratory development and manufacturing campaigns. A combinatory approach outlined herein could be used to provide the optimum conditions for the DARA process. Furthermore, the feasible operating region was mapped out, which highlighted the complexity of the investigated chemistry and aided in developing the control strategy and regulatory submission package pertinent to this reaction. The efforts allowed the process to be successfully validated and scaled using a plug flow reactor (PFR) to manufacture 3200 kg of (S)-7,9-dimethyl-N-(2-methyl-2H-tetrazol-5-yl)-2,3,4,5-tetrahydro-1H-benzo[b]azepin-5-amine under current Good Manufacturing Practice (cGMP).
中文翻译:

钌催化的直接不对称还原胺化反应用于批量或连续工艺放大的机理研究:工业视角
对Ru催化的直接不对称还原胺化(DARA)反应生产活性药物成分(API)中间体进行了全面评估。进行实验以研究工艺参数(例如反应温度,时间,浓度,压力,Ru催化剂浓度,酸催化剂和试剂化学计量)对化学和立体选择性以及收率的影响。对实验数据的分析导致了机械数学模型的开发,该数学模型与实验室开发和制造活动中的数据在数学上是一致的。本文概述的组合方法可用于为DARA过程提供最佳条件。此外,规划了可行的操作区域,这突出了所研究化学的复杂性,并帮助开发了与该反应有关的控制策略和法规提交文件。经过努力,使用塞流反应器(PFR)成功验证了工艺并进行了规模化生产,制造了3200公斤的(S)-7,9-二甲基-N-(2-甲基-2 H-四唑-5-基)-2,3,4,5-四氢-1 H-苯并[ b ]氮杂-5-胺良好生产规范(cGMP)。
更新日期:2017-10-03
中文翻译:

钌催化的直接不对称还原胺化反应用于批量或连续工艺放大的机理研究:工业视角
对Ru催化的直接不对称还原胺化(DARA)反应生产活性药物成分(API)中间体进行了全面评估。进行实验以研究工艺参数(例如反应温度,时间,浓度,压力,Ru催化剂浓度,酸催化剂和试剂化学计量)对化学和立体选择性以及收率的影响。对实验数据的分析导致了机械数学模型的开发,该数学模型与实验室开发和制造活动中的数据在数学上是一致的。本文概述的组合方法可用于为DARA过程提供最佳条件。此外,规划了可行的操作区域,这突出了所研究化学的复杂性,并帮助开发了与该反应有关的控制策略和法规提交文件。经过努力,使用塞流反应器(PFR)成功验证了工艺并进行了规模化生产,制造了3200公斤的(S)-7,9-二甲基-N-(2-甲基-2 H-四唑-5-基)-2,3,4,5-四氢-1 H-苯并[ b ]氮杂-5-胺良好生产规范(cGMP)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号