当前位置:
X-MOL 学术
›
React. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of mixing bio-oil aqueous phase model compounds on hydrogen production in non-catalytic supercritical reforming
Reaction Chemistry & Engineering ( IF 3.4 ) Pub Date : 2017-08-01 00:00:00 , DOI: 10.1039/c7re00090a F. J. Gutiérrez Ortiz 1, 2, 3, 4, 5 , F. J. Campanario 1, 2, 3, 4, 5 , P. Ollero 1, 2, 3, 4, 5
Reaction Chemistry & Engineering ( IF 3.4 ) Pub Date : 2017-08-01 00:00:00 , DOI: 10.1039/c7re00090a F. J. Gutiérrez Ortiz 1, 2, 3, 4, 5 , F. J. Campanario 1, 2, 3, 4, 5 , P. Ollero 1, 2, 3, 4, 5
Affiliation
|
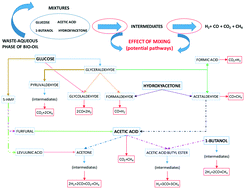
Bio-oil derived from biomass fast pyrolysis can be processed into fuel or some chemical products, but it has a waste aqueous phase that, however, may be valorized. Supercritical reforming of this stream, simulated using mixtures of model compounds (acetic acid, acetol, 1-butanol and glucose), was experimentally studied in a tubular reactor without using a catalyst. The effect of mixing the model compounds at different operating parameters (temperature, feed composition, and residence time) on the process performance was investigated, thus addressing an important chemical aspect of biomass-based renewable energy. The experimental dry gas composition consisted of H2, CO2, CO and CH4, although the gas yields were far from equilibrium. Hydrogen yields were normally less than 2.0 moles of H2 per mole of organic feed, which are lower than those obtained for pure compounds with the same concentration. Based on the analyzed liquid samples, a series of probable reaction pathways were proposed to explain the experimental results by considering the interactions among the compounds and their formed intermediates. Thus, under tested supercritical conditions, the residence time was insufficient to reform the formed methane into hydrogen, thus leading to lower hydrogen production.
中文翻译:

生物油水相模型化合物的混合对非催化超临界重整制氢的影响
可以将源自生物质快速热解的生物油加工成燃料或某些化学产品,但是它具有废水相,但是可以加以利用。在不使用催化剂的情况下,在管式反应器中对使用模型化合物(乙酸,丙酮醇,1-丁醇和葡萄糖)的混合物进行模拟的超临界重整进行了实验研究。研究了在不同的操作参数(温度,进料组成和停留时间)下混合模型化合物对工艺性能的影响,从而解决了基于生物质的可再生能源在化学方面的重要问题。实验干燥气体成分由H 2,CO 2,CO和CH 4组成,尽管天然气产量远未达到平衡。氢气产率通常低于每摩尔有机进料2.0摩尔H 2,这低于具有相同浓度的纯化合物所获得的氢气产率。在分析液体样品的基础上,提出了一系列可能的反应途径,通过考虑化合物及其形成的中间体之间的相互作用来解释实验结果。因此,在测试的超临界条件下,停留时间不足以将形成的甲烷重整为氢,从而导致较低的氢产生。
更新日期:2017-10-03
中文翻译:

生物油水相模型化合物的混合对非催化超临界重整制氢的影响
可以将源自生物质快速热解的生物油加工成燃料或某些化学产品,但是它具有废水相,但是可以加以利用。在不使用催化剂的情况下,在管式反应器中对使用模型化合物(乙酸,丙酮醇,1-丁醇和葡萄糖)的混合物进行模拟的超临界重整进行了实验研究。研究了在不同的操作参数(温度,进料组成和停留时间)下混合模型化合物对工艺性能的影响,从而解决了基于生物质的可再生能源在化学方面的重要问题。实验干燥气体成分由H 2,CO 2,CO和CH 4组成,尽管天然气产量远未达到平衡。氢气产率通常低于每摩尔有机进料2.0摩尔H 2,这低于具有相同浓度的纯化合物所获得的氢气产率。在分析液体样品的基础上,提出了一系列可能的反应途径,通过考虑化合物及其形成的中间体之间的相互作用来解释实验结果。因此,在测试的超临界条件下,停留时间不足以将形成的甲烷重整为氢,从而导致较低的氢产生。











































 京公网安备 11010802027423号
京公网安备 11010802027423号