当前位置:
X-MOL 学术
›
J. Hepatol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
IL-1 receptor like 1 protects against alcoholic liver injury by limiting NF-κB activation in hepatic macrophages
Journal of Hepatology ( IF 26.8 ) Pub Date : 2018-01-01 , DOI: 10.1016/j.jhep.2017.08.023 Meng Wang 1 , Guannan Shen 1 , Liangguo Xu 2 , Xiaodong Liu 3 , Jared M Brown 1 , Dechun Feng 4 , Ruth Ann Ross 5 , Bin Gao 4 , Suthat Liangpunsakul 6 , Cynthia Ju 1
Journal of Hepatology ( IF 26.8 ) Pub Date : 2018-01-01 , DOI: 10.1016/j.jhep.2017.08.023 Meng Wang 1 , Guannan Shen 1 , Liangguo Xu 2 , Xiaodong Liu 3 , Jared M Brown 1 , Dechun Feng 4 , Ruth Ann Ross 5 , Bin Gao 4 , Suthat Liangpunsakul 6 , Cynthia Ju 1
Affiliation
|
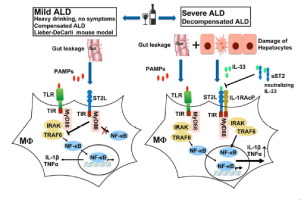
BACKGROUND & AIM
Alcohol consumption increases intestinal permeability and causes damage to hepatocytes, leading to the release of pathogen- and damage-associated molecular pattern molecules (PAMPs and DAMPs), stimulating hepatic macrophages and activating NF-κB. The resultant inflammation exacerbates alcoholic liver disease (ALD). However, much less is known about the mechanisms attenuating inflammation and preventing disease progression in most heavy drinkers. Interleukin (IL)-33 is a DAMP (alarmin) released from dead cells that acts through its receptor, IL-1 receptor like 1 (ST2). ST2 signaling has been reported to either stimulate or inhibit NF-κB activation. The role of IL-33/ST2 in ALD has not been studied. METHODS
Serum levels of IL-33 and its decoy receptor, soluble ST2 (sST2) were measured in ALD patients. Alcohol-induced liver injury, inflammation and hepatic macrophage activation were compared between wild-type, IL-33-/- and ST2-/- mice in several models. RESULTS
Elevation of serum IL-33 and sST2 were only observed in patients with severe decompensated ALD. Consistently, in mice with mild ALD without significant cell death and IL-33 release, IL-33 deletion did not affect alcohol-induced liver damage. However, ST2-deletion exacerbated ALD, through enhancing NF-κB activation in liver macrophages. In contrast, when extracellular IL-33 was markedly elevated, liver injury and inflammation were attenuated in both IL-33-/- and ST2-/- mice compared to wild-type mice. CONCLUSION
Our data revealed a dichotomous role of IL-33/ST2 signaling during ALD development. At early and mild stages, ST2 restrains the inflammatory activation of hepatic macrophages, through inhibiting NF-κB, and plays a protective function in an IL-33-independent fashion. During severe liver injury, significant cell death and marked IL-33 release occur, which triggers IL-33/ST2 signaling and exacerbates tissue damage. LAY SUMMARY
In mild ALD, ST2 negatively regulates the inflammatory activation of hepatic macrophages, thereby protecting against alcohol-induced liver damage, whereas in the case of severe liver injury, the release of extracellular IL-33 may exacerbate tissue inflammation by triggering the canonical IL-33/ST2L signaling in hepatic macrophages.
中文翻译:

IL-1 受体 1 通过限制肝巨噬细胞中 NF-κB 的激活来防止酒精性肝损伤
背景与目的 饮酒会增加肠道通透性并导致肝细胞损伤,导致病原体和损伤相关分子模式分子(PAMP 和 DAMP)的释放,刺激肝巨噬细胞并激活 NF-κB。由此产生的炎症会加剧酒精性肝病(ALD)。然而,对于大多数酗酒者减轻炎症和预防疾病进展的机制知之甚少。白介素 (IL)-33 是一种死亡细胞释放的 DAMP(警报素),通过其受体 IL-1 受体样 1 (ST2) 发挥作用。据报道,ST2 信号传导可刺激或抑制 NF-κB 激活。 IL-33/ST2 在 ALD 中的作用尚未研究。方法 测量 ALD 患者的血清 IL-33 及其诱饵受体可溶性 ST2 (sST2) 水平。在几种模型中比较了野生型、IL-33-/- 和 ST2-/- 小鼠之间酒精引起的肝损伤、炎症和肝巨噬细胞活化。结果 仅在严重失代偿 ALD 患者中观察到血清 IL-33 和 sST2 升高。一致的是,在没有明显细胞死亡和 IL-33 释放的轻度 ALD 小鼠中,IL-33 缺失不会影响酒精引起的肝损伤。然而,ST2 缺失通过增强肝脏巨噬细胞中 NF-κB 的激活而加剧 ALD。相反,当细胞外IL-33显着升高时,与野生型小鼠相比,IL-33-/-和ST2-/-小鼠的肝损伤和炎症均减轻。结论 我们的数据揭示了 IL-33/ST2 信号在 ALD 发展过程中的二分作用。在早期和轻度阶段,ST2通过抑制NF-κB抑制肝巨噬细胞的炎症活化,并以不依赖IL-33的方式发挥保护功能。 在严重肝损伤期间,会发生大量细胞死亡和明显的 IL-33 释放,从而触发 IL-33/ST2 信号传导并加剧组织损伤。在轻度 ALD 中,ST2 负向调节肝巨噬细胞的炎症激活,从而防止酒精引起的肝损伤,而在严重肝损伤的情况下,细胞外 IL-33 的释放可能会通过触发经典 IL 来加剧组织炎症。肝巨噬细胞中的-33/ST2L 信号传导。
更新日期:2018-01-01
中文翻译:

IL-1 受体 1 通过限制肝巨噬细胞中 NF-κB 的激活来防止酒精性肝损伤
背景与目的 饮酒会增加肠道通透性并导致肝细胞损伤,导致病原体和损伤相关分子模式分子(PAMP 和 DAMP)的释放,刺激肝巨噬细胞并激活 NF-κB。由此产生的炎症会加剧酒精性肝病(ALD)。然而,对于大多数酗酒者减轻炎症和预防疾病进展的机制知之甚少。白介素 (IL)-33 是一种死亡细胞释放的 DAMP(警报素),通过其受体 IL-1 受体样 1 (ST2) 发挥作用。据报道,ST2 信号传导可刺激或抑制 NF-κB 激活。 IL-33/ST2 在 ALD 中的作用尚未研究。方法 测量 ALD 患者的血清 IL-33 及其诱饵受体可溶性 ST2 (sST2) 水平。在几种模型中比较了野生型、IL-33-/- 和 ST2-/- 小鼠之间酒精引起的肝损伤、炎症和肝巨噬细胞活化。结果 仅在严重失代偿 ALD 患者中观察到血清 IL-33 和 sST2 升高。一致的是,在没有明显细胞死亡和 IL-33 释放的轻度 ALD 小鼠中,IL-33 缺失不会影响酒精引起的肝损伤。然而,ST2 缺失通过增强肝脏巨噬细胞中 NF-κB 的激活而加剧 ALD。相反,当细胞外IL-33显着升高时,与野生型小鼠相比,IL-33-/-和ST2-/-小鼠的肝损伤和炎症均减轻。结论 我们的数据揭示了 IL-33/ST2 信号在 ALD 发展过程中的二分作用。在早期和轻度阶段,ST2通过抑制NF-κB抑制肝巨噬细胞的炎症活化,并以不依赖IL-33的方式发挥保护功能。 在严重肝损伤期间,会发生大量细胞死亡和明显的 IL-33 释放,从而触发 IL-33/ST2 信号传导并加剧组织损伤。在轻度 ALD 中,ST2 负向调节肝巨噬细胞的炎症激活,从而防止酒精引起的肝损伤,而在严重肝损伤的情况下,细胞外 IL-33 的释放可能会通过触发经典 IL 来加剧组织炎症。肝巨噬细胞中的-33/ST2L 信号传导。











































 京公网安备 11010802027423号
京公网安备 11010802027423号