当前位置:
X-MOL 学术
›
J. Hepatol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
IL-33 exacerbates liver sterile inflammation by amplifying neutrophil extracellular trap formation
Journal of Hepatology ( IF 26.8 ) Pub Date : 2018-01-01 , DOI: 10.1016/j.jhep.2017.09.010 Hamza O Yazdani 1 , Hui-Wei Chen 1 , Samer Tohme 1 , Sheng Tai 2 , Dirk J van der Windt 1 , Patricia Loughran 3 , Brian R Rosborough 1 , Vikas Sud 1 , Donna Beer-Stolz 4 , Heth R Turnquist 5 , Allan Tsung 1 , Hai Huang 6
Journal of Hepatology ( IF 26.8 ) Pub Date : 2018-01-01 , DOI: 10.1016/j.jhep.2017.09.010 Hamza O Yazdani 1 , Hui-Wei Chen 1 , Samer Tohme 1 , Sheng Tai 2 , Dirk J van der Windt 1 , Patricia Loughran 3 , Brian R Rosborough 1 , Vikas Sud 1 , Donna Beer-Stolz 4 , Heth R Turnquist 5 , Allan Tsung 1 , Hai Huang 6
Affiliation
|
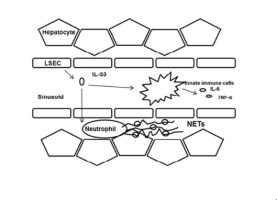
BACKGROUND & AIMS
Neutrophils and liver sinusoidal endothelial cells (LSECs) both contribute to sterile inflammatory injury during ischemia/reperfusion (I/R), a well-known liver surgical stress. Interleukin-33 (IL-33) has been shown to drive neutrophil infiltration during inflammatory responses through its receptor ST2. We recently reported that infiltrating neutrophils form neutrophil extracellular traps (NETs), which exacerbate sterile inflammatory injury in liver I/R. Here, we sought to determine the role of IL-33 in NET formation during liver sterile inflammation. METHODS
Evaluation of IL-33 forming NETs was investigated using a partial liver I/R model to generate sterile injury in healthy WT, IL-33 and ST2 knockouts. Serum levels of IL-33 and myeloperoxidase (MPO)-DNA complex were measured in both humans and mice after the first surgery. Liver damage was assessed. Mouse neutrophil depletion was performed by intraperitoneal injection of anti-Ly6G antibody before I/R. RESULTS
Patients undergoing liver resection showed a significant increase in serum IL-33 compared to healthy volunteers. This coincided with higher serum MPO-DNA complexes. NET formation was decreased in IL-33 and ST2 knockout mice compared with control mice, after liver I/R. IL-33 or ST2 deficiency protected livers from I/R injury, whereas rIL-33 administration during I/R exacerbated hepatotoxicity and systemic inflammation. In vitro, IL-33 is released from LSECs to promote NET formation. IL-33 deficient LSECs failed to induce NETs. ST2 deficient neutrophils limited their capacity to form NETs in vitro and adoptive transfer of ST2 knockout neutrophils to neutrophil-depleted WT mice significantly decreased NET formation. CONCLUSIONS
Data establish that IL-33, mainly released from LSECs, causes excessive sterile inflammation after hepatic I/R by inducing NET formation. Therapeutic targeting of IL-33/ST2 might extend novel strategies to minimize organ damage in various clinical settings associated with sterile inflammation. LAY SUMMARY
Liver ischemia and reperfusion injury results in the formation of neutrophil extracellular traps, which contribute to organ damage in liver surgeries. Herein, we show that IL-33 is released from liver sinusoidal endothelial cells to promote NET formation during liver I/R, which exacerbates inflammatory cascades and sterile inflammation.
中文翻译:

IL-33 通过放大中性粒细胞胞外陷阱的形成而加剧肝脏无菌性炎症
背景和目的 中性粒细胞和肝窦内皮细胞 (LSEC) 都会在缺血/再灌注 (I/R)(一种众所周知的肝脏手术应激)期间导致无菌性炎症损伤。白细胞介素 33 (IL-33) 已被证明可通过其受体 ST2 在炎症反应过程中驱动中性粒细胞浸润。我们最近报道,浸润性中性粒细胞形成中性粒细胞胞外陷阱(NET),从而加剧肝脏缺血再灌注中的无菌性炎症损伤。在这里,我们试图确定 IL-33 在肝脏无菌炎症期间 NET 形成中的作用。方法 使用部分肝脏 I/R 模型对形成 NET 的 IL-33 进行评估,以在健康 WT、IL-33 和 ST2 敲除中产生无菌损伤。第一次手术后,测量了人和小鼠的血清 IL-33 和髓过氧化物酶 (MPO)-DNA 复合物水平。评估肝损伤。在I/R之前通过腹膜内注射抗Ly6G抗体来去除小鼠中性粒细胞。结果 与健康志愿者相比,接受肝切除术的患者血清 IL-33 显着增加。这与较高的血清 MPO-DNA 复合物相一致。与对照小鼠相比,肝脏缺血再灌注后,IL-33 和 ST2 敲除小鼠的 NET 形成减少。 IL-33 或 ST2 缺乏可保护肝脏免受 I/R 损伤,而 I/R 期间施用 rIL-33 会加剧肝毒性和全身炎症。在体外,LSEC 释放 IL-33,促进 NET 形成。 IL-33 缺陷的 LSEC 未能诱导 NET。 ST2缺陷的中性粒细胞限制了它们在体外形成NET的能力,并且将ST2敲除的中性粒细胞过继转移到中性粒细胞耗尽的WT小鼠中显着降低了NET的形成。 结论 数据表明,IL-33 主要由 LSEC 释放,通过诱导 NET 形成,在肝 I/R 后引起过度的无菌性炎症。 IL-33/ST2 的治疗靶向可能会扩展新的策略,以最大限度地减少与无菌炎症相关的各种临床环境中的器官损伤。总结 肝脏缺血和再灌注损伤会导致中性粒细胞胞外陷阱的形成,从而导致肝脏手术中的器官损伤。在此,我们发现 IL-33 从肝窦内皮细胞中释放,促进肝 I/R 期间 NET 的形成,从而加剧炎症级联反应和无菌性炎症。
更新日期:2018-01-01
中文翻译:

IL-33 通过放大中性粒细胞胞外陷阱的形成而加剧肝脏无菌性炎症
背景和目的 中性粒细胞和肝窦内皮细胞 (LSEC) 都会在缺血/再灌注 (I/R)(一种众所周知的肝脏手术应激)期间导致无菌性炎症损伤。白细胞介素 33 (IL-33) 已被证明可通过其受体 ST2 在炎症反应过程中驱动中性粒细胞浸润。我们最近报道,浸润性中性粒细胞形成中性粒细胞胞外陷阱(NET),从而加剧肝脏缺血再灌注中的无菌性炎症损伤。在这里,我们试图确定 IL-33 在肝脏无菌炎症期间 NET 形成中的作用。方法 使用部分肝脏 I/R 模型对形成 NET 的 IL-33 进行评估,以在健康 WT、IL-33 和 ST2 敲除中产生无菌损伤。第一次手术后,测量了人和小鼠的血清 IL-33 和髓过氧化物酶 (MPO)-DNA 复合物水平。评估肝损伤。在I/R之前通过腹膜内注射抗Ly6G抗体来去除小鼠中性粒细胞。结果 与健康志愿者相比,接受肝切除术的患者血清 IL-33 显着增加。这与较高的血清 MPO-DNA 复合物相一致。与对照小鼠相比,肝脏缺血再灌注后,IL-33 和 ST2 敲除小鼠的 NET 形成减少。 IL-33 或 ST2 缺乏可保护肝脏免受 I/R 损伤,而 I/R 期间施用 rIL-33 会加剧肝毒性和全身炎症。在体外,LSEC 释放 IL-33,促进 NET 形成。 IL-33 缺陷的 LSEC 未能诱导 NET。 ST2缺陷的中性粒细胞限制了它们在体外形成NET的能力,并且将ST2敲除的中性粒细胞过继转移到中性粒细胞耗尽的WT小鼠中显着降低了NET的形成。 结论 数据表明,IL-33 主要由 LSEC 释放,通过诱导 NET 形成,在肝 I/R 后引起过度的无菌性炎症。 IL-33/ST2 的治疗靶向可能会扩展新的策略,以最大限度地减少与无菌炎症相关的各种临床环境中的器官损伤。总结 肝脏缺血和再灌注损伤会导致中性粒细胞胞外陷阱的形成,从而导致肝脏手术中的器官损伤。在此,我们发现 IL-33 从肝窦内皮细胞中释放,促进肝 I/R 期间 NET 的形成,从而加剧炎症级联反应和无菌性炎症。










































 京公网安备 11010802027423号
京公网安备 11010802027423号