当前位置:
X-MOL 学术
›
ACS Infect. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Experimental Evolution of Diverse Strains as a Method for the Determination of Biochemical Mechanisms of Action for Novel Pyrrolizidinone Antibiotics
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2017-09-27 00:00:00 , DOI: 10.1021/acsinfecdis.7b00135 Kathryn Beabout 1 , Megan D. McCurry 1 , Heer Mehta 1 , Akshay A. Shah 2 , Kiran Kumar Pulukuri 2 , Stephan Rigol 2 , Yanping Wang 2 , K. C. Nicolaou 2 , Yousif Shamoo 1
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2017-09-27 00:00:00 , DOI: 10.1021/acsinfecdis.7b00135 Kathryn Beabout 1 , Megan D. McCurry 1 , Heer Mehta 1 , Akshay A. Shah 2 , Kiran Kumar Pulukuri 2 , Stephan Rigol 2 , Yanping Wang 2 , K. C. Nicolaou 2 , Yousif Shamoo 1
Affiliation
|
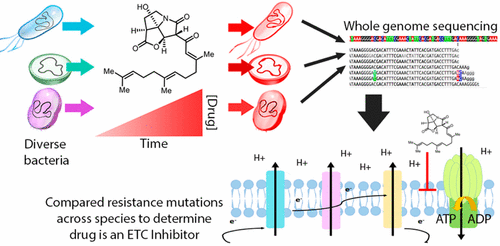
The continuing rise of multidrug resistant pathogens has made it clear that in the absence of new antibiotics we are moving toward a “postantibiotic” world, in which even routine infections will become increasingly untreatable. There is a clear need for the development of new antibiotics with truly novel mechanisms of action to combat multidrug resistant pathogens. Experimental evolution to resistance can be a useful tactic for the characterization of the biochemical mechanism of action for antibiotics of interest. Herein, we demonstrate that the use of a diverse panel of strains with well-annotated reference genomes improves the success of using experimental evolution to characterize the mechanism of action of a novel pyrrolizidinone antibiotic analog. Importantly, we used experimental evolution under conditions that favor strongly polymorphic populations to adapt a panel of three substantially different Gram-positive species (lab strain Bacillus subtilis and clinical strains methicillin-resistant Staphylococcus aureus MRSA131 and Enterococcus faecalis S613) to produce a sufficiently diverse set of evolutionary outcomes. Comparative whole genome sequencing (WGS) between the susceptible starting strain and the resistant strains was then used to identify the genetic changes within each species in response to the pyrrolizidinone. Taken together, the adaptive response across a range of organisms allowed us to develop a readily testable hypothesis for the mechanism of action of the CJ-16 264 analog. In conjunction with mitochondrial inhibition studies, we were able to elucidate that this novel pyrrolizidinone antibiotic is an electron transport chain (ETC) inhibitor. By studying evolution to resistance in a panel of different species of bacteria, we have developed an enhanced method for the characterization of new lead compounds for the discovery of new mechanisms of action.
中文翻译:

多种菌株作为新型吡咯烷酮酮抗生素生化作用机理测定方法的实验进展
耐多药病原体的持续增长表明,在缺乏新抗生素的情况下,我们正朝着“抗生素后世界”迈进,即使常规感染也将越来越难以治愈。显然需要开发具有真正新颖的作用机制以对抗多药耐药性病原体的新型抗生素。对耐药性的实验进化可能是表征目标抗生素作用的生化机制的有用策略。在本文中,我们证明了使用带有丰富注释的参考基因组的多种菌株可以提高使用实验进化来表征新型吡咯烷酮酮抗生素类似物的作用机理的成功率。重要的,枯草芽孢杆菌和临床菌株耐甲氧西林金黄色葡萄球菌MRSA131和粪肠球菌S613),以产生足够多样化的进化结果集。然后,使用敏感的起始菌株和抗性菌株之间的比较全基因组测序(WGS)来鉴定每个物种中对吡咯烷酮的响应的遗传变化。综上所述,跨多种生物体的适应性反应使我们能够为CJ-16 264类似物的作用机理建立易于检验的假设。结合线粒体抑制研究,我们能够阐明这种新型吡咯烷酮酮抗生素是电子传输链(ETC)抑制剂。通过研究一组不同细菌物种中抗药性的进化,我们开发了一种增强的方法,用于表征新的先导化合物,以发现新的作用机理。
更新日期:2017-09-27
中文翻译:

多种菌株作为新型吡咯烷酮酮抗生素生化作用机理测定方法的实验进展
耐多药病原体的持续增长表明,在缺乏新抗生素的情况下,我们正朝着“抗生素后世界”迈进,即使常规感染也将越来越难以治愈。显然需要开发具有真正新颖的作用机制以对抗多药耐药性病原体的新型抗生素。对耐药性的实验进化可能是表征目标抗生素作用的生化机制的有用策略。在本文中,我们证明了使用带有丰富注释的参考基因组的多种菌株可以提高使用实验进化来表征新型吡咯烷酮酮抗生素类似物的作用机理的成功率。重要的,枯草芽孢杆菌和临床菌株耐甲氧西林金黄色葡萄球菌MRSA131和粪肠球菌S613),以产生足够多样化的进化结果集。然后,使用敏感的起始菌株和抗性菌株之间的比较全基因组测序(WGS)来鉴定每个物种中对吡咯烷酮的响应的遗传变化。综上所述,跨多种生物体的适应性反应使我们能够为CJ-16 264类似物的作用机理建立易于检验的假设。结合线粒体抑制研究,我们能够阐明这种新型吡咯烷酮酮抗生素是电子传输链(ETC)抑制剂。通过研究一组不同细菌物种中抗药性的进化,我们开发了一种增强的方法,用于表征新的先导化合物,以发现新的作用机理。











































 京公网安备 11010802027423号
京公网安备 11010802027423号