当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enhanced oxygen exchange of perovskite oxide surfaces through strain-driven chemical stabilization†
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2017-09-27 00:00:00 , DOI: 10.1039/c7ee00770a Bonjae Koo 1, 2, 3 , Hyunguk Kwon 3, 4, 5 , YeonJu Kim 1, 2, 3 , Han Gil Seo 1, 2, 3 , Jeong Woo Han 3, 4, 5 , WooChul Jung 1, 2, 3
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2017-09-27 00:00:00 , DOI: 10.1039/c7ee00770a Bonjae Koo 1, 2, 3 , Hyunguk Kwon 3, 4, 5 , YeonJu Kim 1, 2, 3 , Han Gil Seo 1, 2, 3 , Jeong Woo Han 3, 4, 5 , WooChul Jung 1, 2, 3
Affiliation
|
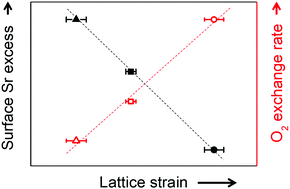
Surface cation segregation and phase separation, of strontium in particular, have been suggested to be the key reason behind the chemical instability of perovskite oxide surfaces and the corresponding performance degradation of solid oxide electrochemical cell electrodes. However, there is no well-established solution for effectively suppressing Sr-related surface instabilities. Here, we control the degree of Sr-excess at the surface of SrTi0.5Fe0.5O3−δ thin films, a model mixed conducting perovskite O2-electrode, through lattice strain, which significantly improves the electrode surface reactivity. Combined theoretical and experimental analyses reveal that Sr cations are intrinsically under a compressive state in the SrTi0.5Fe0.5O3−δ lattice and that the Sr–O bonds are weakened by the local pressure around the Sr cation, which is the key origin of surface Sr enrichment. Based on these findings, we successfully demonstrate that when a large-sized isovalent dopant is added, Sr-excess can be remarkably alleviated, improving the chemical stability of the resulting perovskite O2-electrodes.
中文翻译:

通过应变驱动的化学稳定作用增强钙钛矿氧化物表面的氧交换†
已经提出特别是锶的表面阳离子偏析和相分离是钙钛矿氧化物表面化学不稳定性和固体氧化物电化学电池电极相应性能下降背后的关键原因。但是,没有有效地抑制与Sr有关的表面不稳定性的成熟解决方案。在这里,我们控制混合导电钙钛矿O 2的模型SrTi 0.5 Fe 0.5 O 3- δ薄膜表面的Sr过剩程度。-电极,通过晶格应变,可显着提高电极表面的反应性。合并的理论和实验分析显示,锶阳离子是固有的压缩状态下的SrTi 0.5铁0.5 ø 3- δ晶格和所述锶-O键由围绕锶阳离子局部压力,这是关键原点减弱表面Sr富集。基于这些发现,我们成功地证明了,当添加大尺寸的等价掺杂剂时,可以显着地减轻过量的Sr,从而改善了所得钙钛矿O 2电极的化学稳定性。
更新日期:2017-09-27
中文翻译:

通过应变驱动的化学稳定作用增强钙钛矿氧化物表面的氧交换†
已经提出特别是锶的表面阳离子偏析和相分离是钙钛矿氧化物表面化学不稳定性和固体氧化物电化学电池电极相应性能下降背后的关键原因。但是,没有有效地抑制与Sr有关的表面不稳定性的成熟解决方案。在这里,我们控制混合导电钙钛矿O 2的模型SrTi 0.5 Fe 0.5 O 3- δ薄膜表面的Sr过剩程度。-电极,通过晶格应变,可显着提高电极表面的反应性。合并的理论和实验分析显示,锶阳离子是固有的压缩状态下的SrTi 0.5铁0.5 ø 3- δ晶格和所述锶-O键由围绕锶阳离子局部压力,这是关键原点减弱表面Sr富集。基于这些发现,我们成功地证明了,当添加大尺寸的等价掺杂剂时,可以显着地减轻过量的Sr,从而改善了所得钙钛矿O 2电极的化学稳定性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号