当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mapping the Binding Site for Escitalopram and Paroxetine in the Human Serotonin Transporter Using Genetically Encoded Photo-Cross-Linkers
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2017-09-26 00:00:00 , DOI: 10.1021/acschembio.7b00338 Hafsteinn Rannversson 1 , Jacob Andersen 1 , Benny Bang-Andersen 2 , Kristian Strømgaard 1
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2017-09-26 00:00:00 , DOI: 10.1021/acschembio.7b00338 Hafsteinn Rannversson 1 , Jacob Andersen 1 , Benny Bang-Andersen 2 , Kristian Strømgaard 1
Affiliation
|
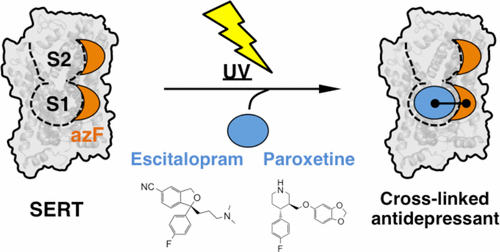
In spite of the important role of the human serotonin transporter (hSERT) in depression treatment, the molecular details of how antidepressant drugs bind are still not completely understood, in particular those related to potential high- and low-affinity binding sites in hSERT. Here, we utilize amber codon suppression in hSERT to encode the photo-cross-linking unnatural amino acid p-azido-l-phenylalanine into the suggested high- and low-affinity binding sites. We then employ UV-induced cross-linking with azF to map the binding site of escitalopram and paroxetine, two prototypical selective serotonin reuptake inhibitors (SSRIs). We find that the two antidepressant drugs exclusively cross-link to azF incorporated at the high-affinity binding site of hSERT, while cross-linking is not observed at the low-affinity binding site. Combined with previous homology models and recent structural data on hSERT, our results provide important information to understand the molecular details of these clinical relevant binding sites.
中文翻译:

映射人类5-羟色胺转运蛋白中的艾司西酞普兰和帕罗西汀的结合位点,使用遗传编码的光-交联剂
尽管人类血清素转运蛋白(hSERT)在抑郁症治疗中起着重要作用,但抗抑郁药如何结合的分子细节仍未完全了解,特别是与hSERT中潜在的高亲和力和低亲和力结合位点有关的分子细节。在这里,我们利用hSERT中的琥珀色密码子抑制来编码光交联非天然氨基酸p -azido- 1-苯丙氨酸进入建议的高亲和力和低亲和力结合位点。然后,我们采用紫外线诱导的与azF的交联,以绘制两种典型的选择性5-羟色胺再摄取抑制剂(SSRIs)依他普仑和帕罗西汀的结合位点。我们发现这两种抗抑郁药专门交联到结合在hSERT高亲和力结合位点处的azF,而在低亲和力结合位点未观察到交联。结合以前的同源性模型和有关hSERT的最新结构数据,我们的结果提供了重要的信息,可帮助您了解这些临床相关结合位点的分子细节。
更新日期:2017-09-26
中文翻译:

映射人类5-羟色胺转运蛋白中的艾司西酞普兰和帕罗西汀的结合位点,使用遗传编码的光-交联剂
尽管人类血清素转运蛋白(hSERT)在抑郁症治疗中起着重要作用,但抗抑郁药如何结合的分子细节仍未完全了解,特别是与hSERT中潜在的高亲和力和低亲和力结合位点有关的分子细节。在这里,我们利用hSERT中的琥珀色密码子抑制来编码光交联非天然氨基酸p -azido- 1-苯丙氨酸进入建议的高亲和力和低亲和力结合位点。然后,我们采用紫外线诱导的与azF的交联,以绘制两种典型的选择性5-羟色胺再摄取抑制剂(SSRIs)依他普仑和帕罗西汀的结合位点。我们发现这两种抗抑郁药专门交联到结合在hSERT高亲和力结合位点处的azF,而在低亲和力结合位点未观察到交联。结合以前的同源性模型和有关hSERT的最新结构数据,我们的结果提供了重要的信息,可帮助您了解这些临床相关结合位点的分子细节。











































 京公网安备 11010802027423号
京公网安备 11010802027423号