当前位置:
X-MOL 学术
›
Chem. Bio. Drug Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural properties of bioactive peptides with α‐glucosidase inhibitory activity
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2017-09-18 , DOI: 10.1111/cbdd.13105 Mohammed Auwal Ibrahim 1, 2 , Megan J. Bester 3 , Albert W. H. Neitz 1 , Anabella R. M. Gaspar 1
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2017-09-18 , DOI: 10.1111/cbdd.13105 Mohammed Auwal Ibrahim 1, 2 , Megan J. Bester 3 , Albert W. H. Neitz 1 , Anabella R. M. Gaspar 1
Affiliation
|
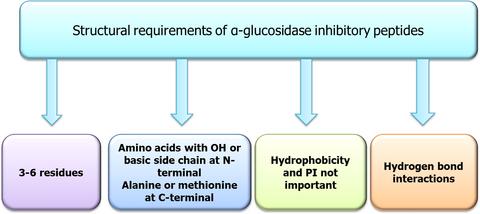
Bioactive peptides are emerging as promising class of drugs that could serve as α‐glucosidase inhibitors for the treatment of type 2 diabetes. This article identifies structural and physicochemical requirements for the design of therapeutically relevant α‐glucosidase inhibitory peptides. So far, a total of 43 fully sequenced α‐glucosidase inhibitory peptides have been reported and 13 of them had IC50 values several folds lower than acarbose. Analysis of the peptides indicates that the most potent peptides are tri‐ to hexapeptides with amino acids containing a hydroxyl or basic side chain at the N‐terminal. The presence of proline within the chain and alanine or methionine at the C‐terminal appears to be relevant for high activity. Hydrophobicity and isoelectric points are less important variables for α‐glucosidase inhibition whilst a net charge of 0 or +1 was predicted for the highly active peptides. In silico simulated gastrointestinal digestion revealed that the high and moderately active peptides, including the most potent peptide (STYV), were gastrointestinally unstable, except SQSPA. Molecular docking of SQSPA, STYV, and STY (digestion fragment of STYV) with α‐glucosidase suggested that their hydrogen bonding interactions and binding energies were comparable with acarbose. The identified criteria will facilitate the design of new peptide‐derived α‐glucosidase inhibitors.
中文翻译:

具有α-葡萄糖苷酶抑制活性的生物活性肽的结构性质
生物活性肽正在成为有希望的一类药物,可以用作治疗2型糖尿病的α-葡萄糖苷酶抑制剂。本文确定了治疗相关的α-葡萄糖苷酶抑制肽设计的结构和物理化学要求。到目前为止,已经报道了43种完全测序的α-葡萄糖苷酶抑制肽,其中13种具有IC 50值比阿卡波糖低几倍。肽段分析表明,最有效的肽段是三肽至六肽,其氨基酸在N端含有羟基或碱性侧链。链中脯氨酸的存在以及C末端的丙氨酸或蛋氨酸的存在似乎与高活性有关。疏水性和等电点对α-葡萄糖苷酶抑制作用不太重要,而高活性肽的净电荷预计为0或+1。在计算机模拟的胃肠道消化中发现,除SQSPA外,高活性和中等活性的肽(包括最有效的肽(STYV))在胃肠道中不稳定。SQSPA,STYV,α-葡萄糖苷酶和STY(STYV的消化片段)表明它们的氢键相互作用和结合能与阿卡波糖相当。确定的标准将有助于设计新的肽源性α-葡萄糖苷酶抑制剂。
更新日期:2017-09-18
中文翻译:

具有α-葡萄糖苷酶抑制活性的生物活性肽的结构性质
生物活性肽正在成为有希望的一类药物,可以用作治疗2型糖尿病的α-葡萄糖苷酶抑制剂。本文确定了治疗相关的α-葡萄糖苷酶抑制肽设计的结构和物理化学要求。到目前为止,已经报道了43种完全测序的α-葡萄糖苷酶抑制肽,其中13种具有IC 50值比阿卡波糖低几倍。肽段分析表明,最有效的肽段是三肽至六肽,其氨基酸在N端含有羟基或碱性侧链。链中脯氨酸的存在以及C末端的丙氨酸或蛋氨酸的存在似乎与高活性有关。疏水性和等电点对α-葡萄糖苷酶抑制作用不太重要,而高活性肽的净电荷预计为0或+1。在计算机模拟的胃肠道消化中发现,除SQSPA外,高活性和中等活性的肽(包括最有效的肽(STYV))在胃肠道中不稳定。SQSPA,STYV,α-葡萄糖苷酶和STY(STYV的消化片段)表明它们的氢键相互作用和结合能与阿卡波糖相当。确定的标准将有助于设计新的肽源性α-葡萄糖苷酶抑制剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号