当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cross-coupling of sulfonic acid derivatives via aryl-radical transfer (ART) using TTMSS or photoredox†
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2017-09-20 00:00:00 , DOI: 10.1039/c7qo00731k Eric D. Nacsa 1, 2, 3, 4 , Tristan H. Lambert 1, 2, 3, 4
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2017-09-20 00:00:00 , DOI: 10.1039/c7qo00731k Eric D. Nacsa 1, 2, 3, 4 , Tristan H. Lambert 1, 2, 3, 4
Affiliation
|
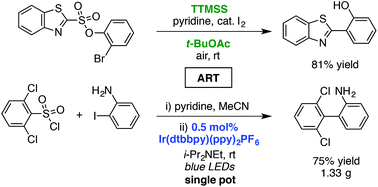
The intramolecular cross-coupling of sulfonic acid derivatives occurs in the presence of tris(trimethylsilyl)silane (TTMSS) at room temperature and in air to form biaryl compounds. A photoredox-catalyzed procedure is also described. These protocols provide mild and convenient alternatives to standard tin-mediated reactions. Combined with the trivial preparation of the substrates from activated sulfonic acids and 2-halophenols or anilines, this work presents a useful means to employ sulfonic acid derivatives in cross-coupling transformations. A modified linker to realize high regioselectivity is also presented. Finally, a one-pot cross-coupling procedure is demonstrated.
中文翻译:

使用TTMSS或光氧化还原通过芳基自由基转移(ART)进行 磺酸衍生物的交叉偶联†
磺酸衍生物的分子内交叉偶联在室温下和在空气中在三(三甲基甲硅烷基)硅烷(TTMSS)存在下发生,以形成联芳基化合物。还描述了光氧化还原催化的方法。这些协议为标准的锡介导反应提供了温和且方便的替代方法。与用活化的磺酸和2-卤代酚或苯胺简单地制备底物相结合,这项工作提供了在交叉偶联转化中使用磺酸衍生物的有用方法。还提出了实现高区域选择性的改进的连接子。最后,演示了一锅交叉耦合程序。
更新日期:2017-09-20
中文翻译:

使用TTMSS或光氧化还原通过芳基自由基转移(ART)进行 磺酸衍生物的交叉偶联†
磺酸衍生物的分子内交叉偶联在室温下和在空气中在三(三甲基甲硅烷基)硅烷(TTMSS)存在下发生,以形成联芳基化合物。还描述了光氧化还原催化的方法。这些协议为标准的锡介导反应提供了温和且方便的替代方法。与用活化的磺酸和2-卤代酚或苯胺简单地制备底物相结合,这项工作提供了在交叉偶联转化中使用磺酸衍生物的有用方法。还提出了实现高区域选择性的改进的连接子。最后,演示了一锅交叉耦合程序。











































 京公网安备 11010802027423号
京公网安备 11010802027423号