当前位置:
X-MOL 学术
›
Chem. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Recent Advances in the Chemical Synthesis of C-Glycosides
Chemical Reviews ( IF 51.4 ) Pub Date : 2017-09-15 00:00:00 , DOI: 10.1021/acs.chemrev.7b00234 You Yang 1 , Biao Yu 2
Chemical Reviews ( IF 51.4 ) Pub Date : 2017-09-15 00:00:00 , DOI: 10.1021/acs.chemrev.7b00234 You Yang 1 , Biao Yu 2
Affiliation
|
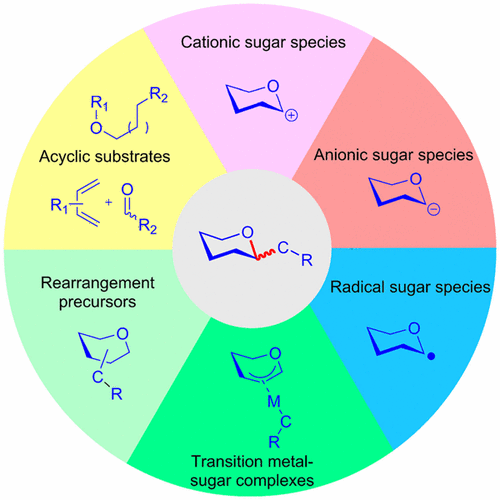
Advances in the chemical synthesis of C-pyranosides/furanosides are summarized, covering the literature from 2000 to 2016. The majority of the methods take advantage of the construction of the glycosidic C—C bond. These C-glycosylation methods are categorized herein in terms of the glycosyl donor precursors, which are commonly used in O-glycoside synthesis and are easily accessible to nonspecialists. They include glycosyl halides, glycals, sugar acetates, sugar lactols, sugar lactones, 1,2-anhydro sugars, thioglycosides/sulfoxides/sulfones, selenoglycosides/telluroglycosides, methyl glycosides, and glycosyl imidates/phosphates. Mechanistically, C-glycosylation reactions can involve glycosyl electrophilic/cationic species, anionic species, radical species, or transition-metal complexes, which are discussed as subcategories under each type of sugar precursor. Moreover, intramolecular rearrangements, such as the Claisen rearrangement, Ramberg–Bäcklund rearrangement, and 1,2-Wittig rearrangement, which usually involve concerted pathways, constitute another category of C-glycosylations. An alternative to the C-glycosylations is the formation of pyranoside/furanoside rings after construction of the predetermined glycosidic C—C bonds, which might involve cyclization of acyclic precursors or D–A cycloadditions. Throughout, the stereoselectivity in the formation of the resultant C-glycosidic linkages is highlighted.
中文翻译:

化学合成C-糖苷的最新进展
总结了2000年至2016年文献中C-吡喃糖苷/呋喃糖苷的化学合成进展。大多数方法都利用糖苷酸C-C键的构建。这些C-糖基化方法根据糖基供体前体在本文中被分类,所述糖基供体前体通常用于O-糖苷合成中并且非专业人员容易获得。它们包括糖基卤化物,糖基,乙酸乙酸酯,糖乳糖醇,糖内酯,1,2-脱水糖,硫代糖苷/亚砜/砜,硒代糖苷/碲代糖苷,甲基糖苷和酰亚胺化的糖基/磷酸盐。机械上,C-糖基化反应可以涉及糖基亲电/阳离子物质,阴离子物质,自由基物质或过渡金属络合物,它们在每种糖前体类型下都作为亚类讨论。此外,通常涉及协同途径的分子内重排,例如克莱森重排,Ramberg-Bäcklund重排和1,2-Wittig重排,构成了另一类C-糖基化。C-糖基化的替代方法是在构建预定的糖苷CC键后形成吡喃糖苷/呋喃糖苷环,这可能涉及无环前体或D-A环加成的环化。整个过程中,形成C的立体选择性-糖苷键突出显示。
更新日期:2017-09-15
中文翻译:

化学合成C-糖苷的最新进展
总结了2000年至2016年文献中C-吡喃糖苷/呋喃糖苷的化学合成进展。大多数方法都利用糖苷酸C-C键的构建。这些C-糖基化方法根据糖基供体前体在本文中被分类,所述糖基供体前体通常用于O-糖苷合成中并且非专业人员容易获得。它们包括糖基卤化物,糖基,乙酸乙酸酯,糖乳糖醇,糖内酯,1,2-脱水糖,硫代糖苷/亚砜/砜,硒代糖苷/碲代糖苷,甲基糖苷和酰亚胺化的糖基/磷酸盐。机械上,C-糖基化反应可以涉及糖基亲电/阳离子物质,阴离子物质,自由基物质或过渡金属络合物,它们在每种糖前体类型下都作为亚类讨论。此外,通常涉及协同途径的分子内重排,例如克莱森重排,Ramberg-Bäcklund重排和1,2-Wittig重排,构成了另一类C-糖基化。C-糖基化的替代方法是在构建预定的糖苷CC键后形成吡喃糖苷/呋喃糖苷环,这可能涉及无环前体或D-A环加成的环化。整个过程中,形成C的立体选择性-糖苷键突出显示。











































 京公网安备 11010802027423号
京公网安备 11010802027423号