当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Optimization of Manganese Coupling Reaction for Kilogram-Scale Preparation of Two Aryl-1,3-dione Building Blocks
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2017-09-08 00:00:00 , DOI: 10.1021/acs.oprd.7b00241 Tomas Smejkal 1 , Vijayagopal Gopalsamuthiram 2 , Sujit K. Ghorai 2 , Anup M. Jawalekar 2 , Dinesh Pagar 2 , Krishna Sawant 2 , Srinivas Subramanian 2 , Jonathan Dallimore 3 , Nigel Willetts 3 , James N. Scutt 3 , Louisa Whalley 3 , Matthew Hotson 3 , Anne-Marie Hogan 3 , George Hodges 3
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2017-09-08 00:00:00 , DOI: 10.1021/acs.oprd.7b00241 Tomas Smejkal 1 , Vijayagopal Gopalsamuthiram 2 , Sujit K. Ghorai 2 , Anup M. Jawalekar 2 , Dinesh Pagar 2 , Krishna Sawant 2 , Srinivas Subramanian 2 , Jonathan Dallimore 3 , Nigel Willetts 3 , James N. Scutt 3 , Louisa Whalley 3 , Matthew Hotson 3 , Anne-Marie Hogan 3 , George Hodges 3
Affiliation
|
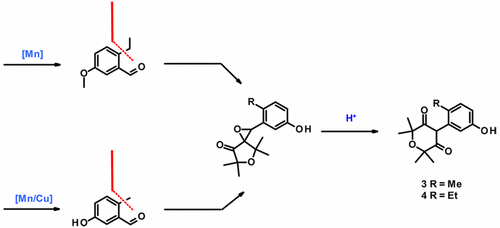
Aryl-1,3-diones represent a promising new class of herbicidal acetyl-CoA carboxylase (ACCase) inhibitors. The original synthesis of this structural motif employed in the research phase involved a selenium oxide mediated oxidation, the use of diazoacetate and aryl lead reagents, and a low temperature oxidation of an aryl lithium intermediate, so it was not well suited to large scale synthesis. For kilogram scale synthesis of the two aryl-1,3-dione building blocks (3 and 4), we developed an alternative route which employs a manganese or manganese–copper catalyzed alkyl Grignard coupling and a semi-pinacol rearrangement of an epoxide as the key steps. The optimized conditions could be of general interest as scalable methods for the synthesis of 2-alkyl substituted benzaldehydes and of 2-aryl-1,3-diones.
中文翻译:

公斤级制备两个芳基1,3-二酮构建基的锰偶联反应的优化
芳基-1,3-二酮代表一种有前途的新型除草乙酰辅酶A羧化酶(ACCase)抑制剂。在研究阶段采用的这种结构基序的原始合成涉及氧化硒介导的氧化,重氮乙酸盐和芳基铅试剂的使用以及芳基锂中间体的低温氧化,因此不适用于大规模合成。对于公斤级合成的两个1,3-芳基芳基结构单元(3和4),我们开发了一种替代方法,该方法采用了锰或锰-铜催化的烷基格利雅(Grignard)偶联和环氧的半频哪醇重排作为关键步骤。作为合成2-烷基取代的苯甲醛和2-芳基-1,3-二酮的可扩展方法,最优化条件可能是普遍感兴趣的。
更新日期:2017-09-08
中文翻译:

公斤级制备两个芳基1,3-二酮构建基的锰偶联反应的优化
芳基-1,3-二酮代表一种有前途的新型除草乙酰辅酶A羧化酶(ACCase)抑制剂。在研究阶段采用的这种结构基序的原始合成涉及氧化硒介导的氧化,重氮乙酸盐和芳基铅试剂的使用以及芳基锂中间体的低温氧化,因此不适用于大规模合成。对于公斤级合成的两个1,3-芳基芳基结构单元(3和4),我们开发了一种替代方法,该方法采用了锰或锰-铜催化的烷基格利雅(Grignard)偶联和环氧的半频哪醇重排作为关键步骤。作为合成2-烷基取代的苯甲醛和2-芳基-1,3-二酮的可扩展方法,最优化条件可能是普遍感兴趣的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号