当前位置:
X-MOL 学术
›
J. Comput. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
σ-holes and π-holes: Similarities and differences
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2017-09-06 , DOI: 10.1002/jcc.24891 Peter Politzer 1 , Jane S. Murray 1
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2017-09-06 , DOI: 10.1002/jcc.24891 Peter Politzer 1 , Jane S. Murray 1
Affiliation
|
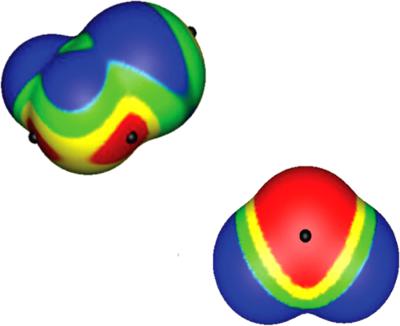
σ‐Holes and π‐holes are regions of molecules with electronic densities lower than their surroundings. There are often positive electrostatic potentials associated with them. Through these potentials, the molecule can interact attractively with negative sites, such as lone pairs, π electrons, and anions. Such noncovalent interactions, “σ‐hole bonding” and “π‐hole bonding,” are increasingly recognized as being important in a number of different areas. In this article, we discuss and compare the natures and characteristics of σ‐holes and π‐holes, and factors that influence the strengths and locations of the resulting electrostatic potentials. © 2017 Wiley Periodicals, Inc.
中文翻译:

σ-holes 和 π-holes:异同
σ 孔和 π 孔是电子密度低于其周围环境的分子区域。通常存在与它们相关的正静电势。通过这些电位,分子可以与负位点发生有吸引力的相互作用,例如孤对、π 电子和阴离子。这种非共价相互作用,即“σ-空穴键合”和“π-空穴键合”,越来越被认为在许多不同领域都很重要。在本文中,我们讨论和比较了 σ 孔和 π 孔的性质和特征,以及影响所产生静电势的强度和位置的因素。© 2017 威利期刊公司。
更新日期:2017-09-06
中文翻译:

σ-holes 和 π-holes:异同
σ 孔和 π 孔是电子密度低于其周围环境的分子区域。通常存在与它们相关的正静电势。通过这些电位,分子可以与负位点发生有吸引力的相互作用,例如孤对、π 电子和阴离子。这种非共价相互作用,即“σ-空穴键合”和“π-空穴键合”,越来越被认为在许多不同领域都很重要。在本文中,我们讨论和比较了 σ 孔和 π 孔的性质和特征,以及影响所产生静电势的强度和位置的因素。© 2017 威利期刊公司。











































 京公网安备 11010802027423号
京公网安备 11010802027423号