当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Anodic Cyclization Reactions and the Mechanistic Strategies That Enable Optimization
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2017-08-31 00:00:00 , DOI: 10.1021/acs.accounts.7b00287 Ruozhu Feng 1 , Jake A. Smith 1 , Kevin D. Moeller 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2017-08-31 00:00:00 , DOI: 10.1021/acs.accounts.7b00287 Ruozhu Feng 1 , Jake A. Smith 1 , Kevin D. Moeller 1
Affiliation
|
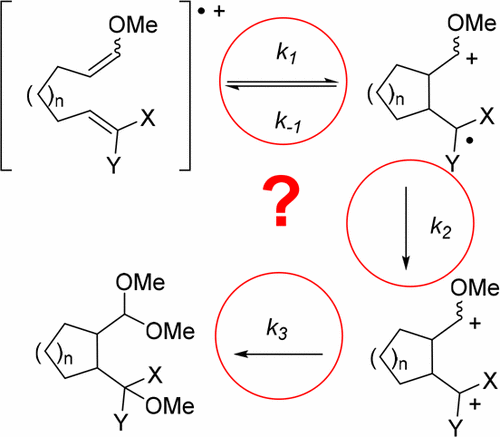
Oxidation reactions are powerful tools for synthesis because they allow us to reverse the polarity of electron-rich functional groups, generate highly reactive intermediates, and increase the functionality of molecules. For this reason, oxidation reactions have been and continue to be the subject of intense study. Central to these efforts is the development of mechanism-based strategies that allow us to think about the reactive intermediates that are frequently central to the success of the reactions and the mechanistic pathways that those intermediates trigger. For example, consider oxidative cyclization reactions that are triggered by the removal of an electron from an electron-rich olefin and lead to cyclic products that are functionalized for further elaboration. For these reactions to be successful, the radical cation intermediate must first be generated using conditions that limit its polymerization and then channeled down a productive desired pathway. Following the cyclization, a second oxidation step is necessary for product formation, after which the resulting cation must be quenched in a controlled fashion to avoid undesired elimination reactions. Problems can arise at any one or all of these steps, a fact that frequently complicates reaction optimization and can discourage the development of new transformations. Fortunately, anodic electrochemistry offers an outstanding opportunity to systematically probe the mechanism of oxidative cyclization reactions. The use of electrochemical methods allows for the generation of radical cations under neutral conditions in an environment that helps prevent polymerization of the intermediate. Once the intermediates have been generated, a series of “telltale indicators” can be used to diagnose which step in an oxidative cyclization is problematic for less successful transformation. A set of potential solutions to address each type of problem encountered has been developed. For example, problems with the initial cyclization reaction leading to either polymerization of the radical cation, elimination of a proton from or solvent trapping of that intermediate, or solvent trapping of the radical cation can be identified in the proton NMR spectrum of the crude reaction material. Such an NMR spectrum shows retention of the trapping group. The problems can be addressed by tuning the radical cation, altering the trapping group, or channeling the reactive intermediate down a radical pathway. Specific examples each are shown in this Account. Problems with the second oxidation step can be identified by poor current efficiency or general decomposition in spite of cyclic voltammetry evidence for a rapid cyclization. Solutions involve improving the oxidation conditions for the radical after cyclization by either the addition of a properly placed electron-donating group in the substrate or an increase in the concentration of electrolyte in the reaction (a change that stabilizes the cation generated from the second oxidation step). Problems with the final cation typically lead to overoxidation. Solutions to this problem require an approach that either slows down elimination side reactions or changes the reaction conditions so that the cation can be quickly trapped in an irreversible fashion. Again, this Account highlights these strategies along with the specific experimental protocols utilized.
中文翻译:

阳极环化反应及实现优化的机理策略
氧化反应是合成的强大工具,因为它们使我们能够反转富含电子的官能团的极性,生成高反应性的中间体并增加分子的功能。由于这个原因,氧化反应已经并且继续是深入研究的主题。这些努力的核心是基于机制的策略的发展,使我们能够考虑反应性中间体,这些中间体通常是反应成功和这些中间体触发的机理途径的核心。例如,考虑氧化环化反应,该反应是通过从富含电子的烯烃中去除电子而触发的,并导致官能化以进一步精制的环状产物。为了使这些反应成功,自由基阳离子中间体必须首先使用限制其聚合的条件生成,然后通过一条生产所需的途径进行引导。环化之后,第二氧化步骤对于产物形成是必需的,在此之后,必须以受控方式将所得阳离子淬灭,以避免不希望的消除反应。这些步骤中的任何一个或所有步骤都可能出现问题,这一事实通常会使反应优化变得复杂,并且会阻碍新转化的发展。幸运的是,阳极电化学为系统地探索氧化环化反应的机理提供了一个绝好的机会。电化学方法的使用允许在中性条件下在有助于防止中间体聚合的环境中产生自由基阳离子。一旦生成了中间体,就可以使用一系列“叙述性指示剂”来诊断氧化环化中的哪个步骤对于转化不太成功是有问题的。已经开发出一套解决每种遇到的问题的潜在解决方案。例如,可以在粗反应材料的质子NMR光谱中识别出初始环化反应导致自由基阳离子聚合,从该中间体消除质子或溶剂捕集该中间体或溶剂捕获自由基阳离子的问题。 。这样的NMR光谱显示了捕获基团的保留。这些问题可以通过调整自由基阳离子,改变捕获基团或将反应性中间体沿着自由基途径引导来解决。每个特定示例都显示在此帐户中。尽管循环伏安法可证明快速环化,但仍可通过不良的电流效率或一般分解来确定第二氧化步骤存在的问题。解决方案包括通过在基板中添加适当放置的供电子基团或增加反应中电解质的浓度(环化后稳定第二个氧化步骤生成的阳离子)来改善环化后自由基的氧化条件)。最终阳离子的问题通常会导致过度氧化。为了解决该问题,需要一种减慢消除副反应或改变反应条件的方法,从而可以以不可逆的方式快速捕获阳离子。再次,
更新日期:2017-08-31
中文翻译:

阳极环化反应及实现优化的机理策略
氧化反应是合成的强大工具,因为它们使我们能够反转富含电子的官能团的极性,生成高反应性的中间体并增加分子的功能。由于这个原因,氧化反应已经并且继续是深入研究的主题。这些努力的核心是基于机制的策略的发展,使我们能够考虑反应性中间体,这些中间体通常是反应成功和这些中间体触发的机理途径的核心。例如,考虑氧化环化反应,该反应是通过从富含电子的烯烃中去除电子而触发的,并导致官能化以进一步精制的环状产物。为了使这些反应成功,自由基阳离子中间体必须首先使用限制其聚合的条件生成,然后通过一条生产所需的途径进行引导。环化之后,第二氧化步骤对于产物形成是必需的,在此之后,必须以受控方式将所得阳离子淬灭,以避免不希望的消除反应。这些步骤中的任何一个或所有步骤都可能出现问题,这一事实通常会使反应优化变得复杂,并且会阻碍新转化的发展。幸运的是,阳极电化学为系统地探索氧化环化反应的机理提供了一个绝好的机会。电化学方法的使用允许在中性条件下在有助于防止中间体聚合的环境中产生自由基阳离子。一旦生成了中间体,就可以使用一系列“叙述性指示剂”来诊断氧化环化中的哪个步骤对于转化不太成功是有问题的。已经开发出一套解决每种遇到的问题的潜在解决方案。例如,可以在粗反应材料的质子NMR光谱中识别出初始环化反应导致自由基阳离子聚合,从该中间体消除质子或溶剂捕集该中间体或溶剂捕获自由基阳离子的问题。 。这样的NMR光谱显示了捕获基团的保留。这些问题可以通过调整自由基阳离子,改变捕获基团或将反应性中间体沿着自由基途径引导来解决。每个特定示例都显示在此帐户中。尽管循环伏安法可证明快速环化,但仍可通过不良的电流效率或一般分解来确定第二氧化步骤存在的问题。解决方案包括通过在基板中添加适当放置的供电子基团或增加反应中电解质的浓度(环化后稳定第二个氧化步骤生成的阳离子)来改善环化后自由基的氧化条件)。最终阳离子的问题通常会导致过度氧化。为了解决该问题,需要一种减慢消除副反应或改变反应条件的方法,从而可以以不可逆的方式快速捕获阳离子。再次,










































 京公网安备 11010802027423号
京公网安备 11010802027423号